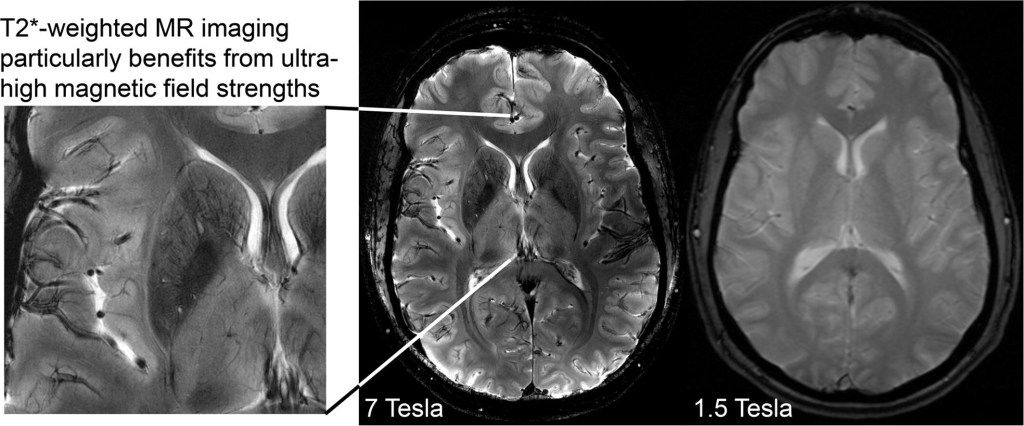
I have already revealed Elizabeth’s scans which were done in November at Lincoln County Hospital with the result of “normal”. I would urge all of my readers to double check what scanner has been used as a 1.5 scanner has not picked up what is clearly visible on the private scans I had done through S G Radiology. I am waiting for an explanation as to what the images portray as I cannot accept these images to be normal. They even state “trauma” so as a parent or carer it is evident that you cannot always assume that normal means normal. A while back Elizabeth had an ultrasound as there were fears of cancer. Now I want all the scans done again under a Tesla 3. I have heard a Tesla 7 is even better and will look into where this can be done in the UK. So Lincolnshire, Enfield, Weston are just three examples where they do not have the up to date scanner and therefore you cannot rely on the results. This affects not just people under MH but everyone.
For so long now I have been trying to get answers – it clearly states “Anterior Region Medial Temperol Compromise which is not a mental illness and yet Elizabeth has been treated under MH with massive dosage of antipsychotics that do not work and is “treatment resistant” – poor/non metaboliser which I have prove by P450 tests. Now everything is falling into place and it is something I want to share with all of you especially those going through cancer treatment. Never trust the word “normal” and check what scanner your Trust has. I am currently asking my MP, Victoria Atkins why Lincolnshire does not have a scanner that is reliable. This means all of Elizabeth’s treatment may be affected as last year there was a cancer scare. Now I want all the scans/ultrasounds done through Sheffield and for Elizabeth to be referred to a Neurological Ward where for once her physical health can be properly assessed over a period of weeks. If the MH team stand in my daughter’s way of her physical health appointments and pathological tests I will report everything on here. I have already requested reports from all the professional bodies who have done nothing so far to stop the abuse to my daughter. Most importantly doctors should put the physical health of their patients first and foremost. I have now highlighted how patients are being deprived under just one area and how patients could have lost their lives due to the scans and how patients under the MH are refused pathological tests. This is why I was banned for months on end visiting my daughter and bullied by various doctors and this needs to be looked into. Police time has been wasted. Having lost close friends to cancer I intend to give this massive publicity.
LPFT under MH do not use PANSS??? Below is what I have written to Lincolnshire Partnership Trust as I am concerned for all the patients on Castle and Ellis Ward who may not have had reliable scans and I do not think anyone is being treated properly and wish to share so that everyone can be aware of the true facts:
PANSS
The PANSS score is an essential diagnostic tool which LPFT are not using and should be. All the scans may need to be re-done under scanners in Sheffield not just the MRI – everything and you may have to review all the patients on both Castle and Ellis Wards who have had scans done in Lincoln under the 1.5 Tesla in light of the revelations below.
The PANSS score is a long established test approved by the Royal College of Psychiatrists and used by NHS Mental Health Teams in many of the trusts to determine the severity of the schizophrenia the patient is suffering from. Its purpose is to confirm diagnosis, to guide the treatment regimen and to determine suitability for matters such as section 17 leave, suitability of the patient for post-discharge accommodation and eventual discharge from mental health care.
Here are LPFT’s comments below:
“LPFT does not use the PANSS scale for various reasons but does use the Glasgow Antipsychotic side effect scale (GASS). This measures the side effects of antipsychotics rather than the efficacy of antipsychotics”.
This is totally unsatisfactory for the following reasons. To begin with to quote LPFT’s own words “for various reasons” gives no explanation at all why this scale is not used by LPFT and is a cursory and unhelpful excuse.
The PANSS scale as mentioned above is crucial in determining not only treatment but deprivation of liberty and the ability of Elizabeth to enjoy leave and to have quality time off the ward. Elizabeth’s stifling detention without hope of even the chance to have a day out or to spend time with her family on important occasions like Christmas and her birthday are a contribution to her state of mind and a detriment to her eventual recovery.
The scale is also used to determine an appropriate treatment regimen which goes beyond the simple use or rapid tranquillisation and isolation preferred by the staff of the hospital in which she is detained. The hospital has obstructed all attempts to have Elizabeth’s mental health condition based on a determination that their ‘diagnosis ‘ of schizophrenia would be the only option. Elizabeth is now seeing a neurologist but that has only happened because that intervention was sought by me and was indeed actively discouraged and obstructed by the Responsible Clinician and the Clinical Lead on the ward.
The Glasgow Antipsychotic Side effect (GASS) scale that LPFT refer to in their email has an entirely different purpose to the PANSS scale and you are well aware of that. The GASS effect scale is for detecting adverse drug reactions and it has to be said if they are indeed using this they have ignored a number of these over the last two and a half years in spite of them being pointed out to them.
LPFT are fully aware that the GASS scale is not a substitute for PANSS but an entirely different test and the reply to the NR was disingenuous and unhelpful. To date no detailed explanation of why PANSS is not being used has been given and the Mental Health Act Team (notably the email does not identify its author) have failed to help once again. Please explain.
In the absence of proper monitoring by the people entrusted with Elizabeth’s care is it hardly surprising that after two and a half years subjected to a deprivation of liberty regime more restrictive than a section 37/41 order and constant prn rapid tranquillisation that she has made no improvement and is as far away from discharge as she ever has been.
The fact is the private scans are most certainly more superior to theirs and have been shown to numerous experts who are biomedical scientists yet they agree. If LPFT hadn’t spent a fortune getting rid of me as NR they would have more money to spend on decent scanners such as Tesla 3 and not having one is putting everyone’s life at risk not just those under MH. The taking away of my daughter’s right of capacity and autonomy is the biggest violation of all human rights. About 10 doctors apart from Dr Memons of Cygnet have stood in the way of her having MRI scans so that is why I was suspicious and arranged it myself. The MH system is ridiculous and I was the best NR and they got rid of me for their own convenience which again is abrogation of my daughter’s human rights. As highlighted in Medscape it is mentioned about brain tumours being missed and metastacising before being found which is potentially fatal which is why I am warning everyone not to trust their NHS scans and to check on which scanner has been used by their Trust.
It is also important to get brain lipids tested. They can affect both metabolising enzymes and result in ADRs. Several endocrine/metabolising disorders are associated with elevated levels of lipids (hyperlipidemia).
RESPONSIBLE CLINICIANS ARE SUPPOSED TO FOLLOW THE MHA 1983 BUT UNDER LPFT WHAT IS BEING DONE IS NOT CONDUCIVE TO ELIZABETH’S RECOVERY AND I WILL FEATURE MORE SHOCKING REVELATIONS ON MY NEXT BLOG. THEY NEEDED TO ROB HER OF HER CAPACITY SO THAT THEY COULD ABUSE THE CONCEPT OF THERAPEUTIC NECESSITY WHILST LABELLED WITH NO CAPACITY. I HAVE CAUGHT ON TO THEIR CRAFTINESS AS THIS GETS THEM OUT OF CONSULTING WITH HER OR RECORDING HER WISHES AND THIS IS WHY THEY GOT RID OF ME AS NR AND THIS IS TO PROTECT THEMSELVES. NO HUMAN RIGHTS AT ALL ARE TAKEN INTO ACCOUT BY LPFT. THE TERRIBLE DENIAL OF LIFE EXPERIENCE IS DETRIMENTAL TO MY DAUGHTER’S MENTAL AND PHYSICAL HEALTH AND TOTAL ABROGATION OF MEDICAL ETHICS. EVEN A S37/41 PATIENT IS NOT DENIED SUCH RIGHTS. HOW CAN HER DETENTION BE LAWFUL UNDER MHA 1983. THEY CONTINUALLY FLOUT THE STATUTORY SAFEGUARDS. THEY SHOULD BE SUBJECTED TO JUDICIAL REVIEW AND PROFESSIONAL MISCONDUCT ACTIONS.
Why 3T is necessary for detecting lesions
- ■ In more than 500 follow-up images, only four of 1996 new or enlarged multiple sclerosis lesions would have been missed with 3.0-T MRI without the administration of contrast material.
- ■ With 3.0-T MRI, the assessment of interval progression did not differ between contrast-enhanced and nonenhanced images.
Introduction
Inflammatory lesions in multiple sclerosis (MS) are detected as focal areas of high signal intensity on T2-weighted MR images. By depicting newly occurring lesions, MRI reveals subclinical disease activity. Therefore, regular follow-up MRI is considered a mainstay of clinical care for patients with MS or clinically isolated syndromes.
Earlier studies have reported that the administration of contrast material is necessary to maximize sensitivity for detecting new lesions. However, these results date back more than 2 decades and were based on two-dimensional images obtained with 4–5-mm-thick sections at magnetic field strengths of 1.5 T and lower.
MRI units with higher field strengths have become widely available, especially for brain imaging. In addition, three-dimensional isotropic MRI sequences were introduced and were shown to outperform conventional two-dimensional sequences in lesion depiction; they are therefore part of recommended MRI standards in MS . Furthermore, the double inversion-recovery (DIR) sequence was introduced. Although this sequence is best known for its ability to depict cortical lesions, it is also useful for depicting white matter lesions Recently, longitudinal subtraction techniques have been developed that show new or enlarged lesions as bright spots while pre-existing lesions and normal-appearing brain parenchyma are canceled out.* Such techniques substantially improve the sensitivity in the detection of new or enlarged lesions in MS at follow-up imaging.
We hypothesized that the use of contrast material does not improve sensitivity in the detection of new or enlarged lesions at follow-up MRI when modern three-dimensional sequences performed at a field strength of 3.0 T are used together with longitudinal subtraction maps. We therefore performed this study to investigate whether the use of contrast material has an effect on the detection of new or enlarged MS lesions and, consequently, the assessment of interval progression.
*The private 3T scans are much more able to see the lesions that the inappropriate 1.5T scanner cannot see.
The higher resolution scans cancel out the distortions and signal noise that hide the lesion and make the scan look normal.
1.5T MRI scanners are not fit for purpose in discovering brain lesions and inflammation.
Brain lesions and inflammation, especially in the temporal lobe are responsible for poor drug response and in some cases for diagnosis.
1.5T scans are responsible for false negative (normal) brain images.
Neuro Second Opinion Catches Missed Brain Lesions Suggestive of MS
Women’s Imaging • Original Report AJR 2010; 194:1674–1679 0361–803X/10/1946–1674 ©
American Roentgen Ray Society Dynamic contrast-enhanced MRI (DCE-MRI) has clearly been shown to be a highly sensitive tool for the detection of breast cancer [1–14]. Reported high sensitivity (83– 100%) [1–5] of MRI for breast cancer led im[1]agers initially to presume that non-enhancing legions on MRI were benign and did not warrant biopsy [1, 15]. However, subsequently reported articles have shown that all malignant lesions do not show enhancement at DCE[1]MRI [2, 5, 16–20], with enhancement absent in up to 12% of known malignant lesions. In a recent multi-institutional study of 995 lesions in 854 women, Schnall et al. [20] reported that 16% of 77 ductal carcinoma in situ (DCIS) lesions and 3% of 422 invasive cancers showed no enhancement. Teifke et al. [18] found 28 (8.4%) of 334 invasive cancers and 13 (65%) of 20 non-invasive cancers were missed at MRI. The reasons given for lack of visualization of these missed lesions were technical difficulties, reader percep[1]Keywords: breast cancer, DCE-MRI, dynamic contrast-enhanced MRI DOI:10.2214/AJR.09.3568 Received September 3, 2009; accepted after revision December 8, 2009.
R. A. Schmidt is a minor stockholder in Hologic Inc., and his spouse receives grant support from Philips Healthcare. G. M. Newstead receives grant support from Philips Healthcare, and her spouse is a minor stockholder in Hologic Inc. WOMEN ’ S I M A G I N G OBJECTIVE.
The objective of our study was to determine the sensitivity of cancer detection at breast MRI using current imaging techniques and to evaluate the characteristics of lesions with false-negative examinations. MATERIALS AND METHODS. Two hundred seventeen patients with 222 newly diagnosed breast cancers or highly suspicious breast lesions that were subsequently shown to be malignant underwent breast MRI examinations for staging. Two breast imaging radiologists performed a consensus review of the breast MRI examinations. The absence of perceptible contrast enhancement at the expected site was considered to be a false-negative MRI. Histology of all lesions was reviewed by an experienced breast pathologist. RESULTS. Enhancement was observed in 213 (95.9%) of the 222 cancer lesions. Of the nine lesions without visible enhancement, two lesions were excluded because the entire tumor had been excised at percutaneous biopsy performed before the MRI examination and no residual tumor was noted on the final histology. The overall sensitivity of MRI for the known cancers was 96.8% (213/220); for invasive cancer, 98.3% (176/179); and for ductal carcinoma in situ, 90.2% (37/41). CONCLUSION.
In a population of 220 sequentially diagnosed breast cancer lesions, we found seven (3.2%) MRI-occult cancers, fewer than seen in other published studies. Small tumor size and diffuse parenchymal enhancement were the principal reasons for these false[1]negative results. Although the overall sensitivity of cancer detection was high (96.8%), it should be emphasized that a negative MRI should not influence the management of a lesion that appears to be of concern on physical examination or on other imaging techniques. Shimauchi et al. MRI-Occult Breast Cancers Women’s Imaging Original Report Downloaded from ajronline.org by 90.246.25.176 on 02/27/24 from IP address 90.246.25.176. Copyright ARRS. For personal use only; all rights reserved AJR:194, June 2010 1675 MRI-Occult Breast Cancers stitution. Forty-four lesions in 44 patients were excluded because the final pathology at lumpectomy or mastectomy was performed at other institutions and was not available for review by our pathologists. The remaining 222 cancers in 217 patients were the subject of this retrospective review. The patients ranged in age from 28 to 85 years (median, 58 years). The spectrum of malignancy size and histology in these 217 patients is shown in Table 1. Of the 222 cancers studied, 84 (37.8%) lesions were palpable, 80 (36.0%) lesions were non-palpable, and clinical data as to palpability was not available for 57 (25.7%) lesions; 209 (94.1%) lesions were visible at mammography, 10 (4.5%) lesions were not visible at mammography, and mammograms were not available for three (1.4%) lesions. Additionally, 166 (74.8%) lesions were visible at sonography, 16 (7.2%) lesions were not visible at sonography, and ultrasound images were not available for 40 (18.0%) lesions. All index cancers, except 32 of 222 lesions, had been diagnosed by percutaneous biopsy or fine-needle aspiration (FNA) before MRI. The remaining 32 cancers underwent MRI because other imaging and clinical findings suggested a highly suspicious lesion and subsequent tissue sampling proved malignancy. Conventional Diagnostic Imaging In all but three patients, mammograms (obtained with different equipment and films, many from different referral centers) were available and were reviewed at our institution by breast imaging specialist radiologists before MRI. Mammograms obtained at outside referral centers accounted for approximately 20% of the total 217 patients. All patients with invasive cancer or extensive DCIS lesions (166 cases, 75%) underwent target[1]ed ultrasound examination of the affected breast and the ipsilateral axillary region, using an ATL HDI 5000 unit (Philips Healthcare International) and a 5-12–MHz linear transducer, before MRI. These imaging examinations were performed by breast specialist radiologists at our institution. An additional ultrasound examination was not standard procedure for patients with a diagnosis of pure DCIS at our institution unless mammograms showed extensive disease suspicious for the presence of an invasive component. MRI Protocol MRI examinations were performed using two 1.5-T imaging units (Signa, GE Healthcare, for 116 patients; and Intera Achieva, Philips Healthcare, for 101 patients). All patients underwent MRI in the prone position using parallel imaging technique. A dedicated 8-channel breast coil was used for the Signa scanner, and a dedicated 7-channel breast coil was used for the Intera Achieva scanner. After obtaining bilateral non-fat-saturated T2-weighted images (TR/TE: Signa, 5,000/103.5; Intera Achieva, 16,907/120) of the breasts, a T1-weighted 3D gradient-echo sequence was performed before and 20 seconds after the injection of contrast material. For the Signa scanner, the imaging parameters were as follows: 4.6/2.2; flip angle, 10°; field of view (FOV), 34 × 34 cm; matrix, 320 × 320; section thickness, 2 mm; and acquisition time, 75 sec[1]onds. For the Intera Achieva scanner, the parameters were as follows: 7.9/3.9; flip angle, 10°; FOV, 48 × 48 cm; matrix, 352 × 352; section thickness, 2 mm; and acquisition time, 75 seconds. A dynamic study in the axial plane was performed six times after initiation of an IV injection of 0.1 mmol/kg of gadodiamide (Omniscan, GE Healthcare) at a rate of 2 mL/s, which was followed by a 20-mL saline flush at the rate of 2 mL/s. MRI examinations were processed by CADstream, version 4.1 (Confirma), and subtraction images, time course curves, and angiogenesis maps were obtained. The images were transferred to a workstation (Advantage Windows, software version 4.0, GE Healthcare) for analysis. Image Analysis IRB-approved retrospective review by consensus was performed by two radiologists with expertise in breast MRI, one with 15 years’ experience and the other with 5 years. Images were interpreted with the benefit of a brief clinical history, knowledge of the histopathologic findings, and knowledge of the mammography and sonography results. In addition to the original images, maximum-intensity-projection and multiplanar reconstruction images were reviewed. The reviewers called findings positive even when there was no color on angiogenesis maps or when, for example, there was a persistent type of curve; they relied on morphology for diagnosis in these cases because TABLE 1: Pathologic Diagnosis in 217 Patients With 222 Malignancies Diagnosis No. (%) of Malignant Lesions No. (%) of Malignant Lesions by Size < 2 cm 2–5 cm > 5 cm Not Available Invasive ductal carcinoma 160 (72) 94 (80) 54 (76) 11 (55) 0 (0) Invasive lobular carcinoma 19 (8) 8 (7) 9 (13) 2 (10) 0 (0) Ductal carcinoma in situ 44 (20) 15 (13) 8 (11) 7 (35) 14 (100) Total 222 (100) 117 (100) 71 (100) 20 (100) 14 (100) TABLE 2: False-Negative Lesions at Dynamic Contrast-Enhanced MRI Case No. Patient Age (y) Pathology Imaging Reason for False[1]Type of Cancer Nuclear Grade Size (mm) Mammography Sonography Size (mm) Negative MRI 1 46 Extensive DCIS 2 NA Pleomorphic and linear calcifications NA 30 Diffuse parenchymal enhancement 2 43 DCIS 3 30 Pleomorphic calcifications Negative 25 Diffuse parenchymal enhancement 3 54 Multifocal DCIS 3 NA Punctate calcifications NA 5 Unknown 4 79 DCIS 1 5 Pleomorphic calcifications NA 5 Small size 5 53 IDC with DCIS 3 IDC, 0.8; DCIS, 10 Amorphous calcifications NA 10 Unknown 6 49 IDC with extensive DCIS 2 IDC, 8; DCIS, NA Pleomorphic and linear calcifications Hypoechoic masses 65 Diffuse parenchymal enhancement 7 44 IDC with DCIS 2 IDC, 12; DCIS, NA Negative Mixed echogenic mass 10 Unknown; low-signal mass on T2-weighted images Median 46 10 Note—DCIS = ductal carcinoma in situ, IDC = invasive ductal carcinoma, NA = not applicable. Downloaded from ajronline.org by 90.246.25.176 on 02/27/24 from IP address 90.246.25.176. Copyright ARRS. For personal use only; all rights reserved 1676 AJR:194, June 2010 Shimauchi et al. some malignant lesions especially DCIS and in[1]vasive lobular carcinoma (ILC) lesions can show slow uptake and perceptible kinetics [21, 22]. The absence of perceptive contrast enhancement at the expected site of the lesion was considered to be a false-negative MRI. Each study was also evaluat[1]ed with bilateral breast parenchymal enhancement scores of minimal, mild, moderate, or marked enhancement by consensus opinion. Technical factors such as patient motion, poor fat suppression, or susceptibility artifacts caused by percutaneously placed metallic clips did not compromise any of the study data sets. Histopathologic Correlation Of the 222 malignant cases, mastectomy was performed for 92 (41%) cases and breast-conserving surgery lumpectomy, with or without needle localization guidance, was performed for 130 cas[1]es (59%). Histology results of all lesions were re[1]viewed by an experienced breast pathologist with 25 years of experience in breast pathology. Tumor type, grade, size, and histologic subtype were documented in the pathology reports. In general, in our pathology department, extensive DCIS is defined as a lesion larger than 5 cm. Correlation between imaging and pathology for each case with regard to location and size and treatment management decisions were discussed for each case at weekly inter[1]nal multidisciplinary breast conference. Results Of the 222 cancerous lesions, enhancement was observed in 213 lesions (95.9%). There were nine examinations that showed no enhancement on MRI. All nine patients had undergone core biopsy or FNA before the MRI examination. Two of the nine lesions were excluded from our false-negative analysis, both presented with a cluster of calcifications, because the entire tumor had been excised by stereotactic core biopsy performed before the MRI examination. In these cases, no residu[1]al tumor was noted at histology on the sub[1]sequent excised breast specimens. Histology of both lesions indicated low-grade DCIS. The sensitivity of MRI for the known cancers was, therefore, 96.8% (213/220) for all cancer, 98.3% (176/179) for invasive cancer, and 90.2% (37/41) for DCIS. Of the 217 patients, 35 patients (16.1%) were recalled for MRI-directed ultrasound, 22 patients (10.1%) underwent ultrasound biopsies with benign results, and nine patients (4.1%) underwent MRI biopsies with benign results. The seven (3.2%) nonenhancing cancers included four cases of DCIS (one low-grade, one intermediate-grade, two high-grade), and three cases of invasive ductal carcinoma (IDC) (two intermediate-grade, one high-grade), as shown in Table 2. One of the two scanners was used for 116 patients (119 lesions), and four malignant lesions (3.4%) were missed. The other scanner was used for 101 patients (103 lesions), and three lesions (2.9%) were missed. All of the false-negative lesions except one were detected at mammography. The mammographic features of these lesions were classified as pleomorphic and linear calcifications (2/6), pleomorphic calcifica[1]tions (2/6), amorphous calcifications (1/6), and punctate calcifications (1/6). One lesion was detected as a palpable, tender mass in the retroareolar region and was visible at sonog[1]raphy but not at mammography. The overall A C Fig. 1—79-year-old woman with history of right lumpectomy for ductal carcinoma in situ (DCIS) 10 years earlier (case 4 in Table 2). A, Craniocaudal magnification view of right breast from routine follow-up mammography shows 5-mm cluster of pleomorphic calcifications (arrow) near lumpectomy scar. Stereotactic core biopsy revealed DCIS, grade 1. B, Craniocaudal mammographic view of right breast obtained after biopsy shows metallic clip with a few residual calcifications (arrow) at site of biopsy. C, T2-weighted image shows susceptibility artifact from metallic clip (arrow). D, On early phase of dynamic contrast-enhanced study (subtraction, T1-weighted image), no enhancement is seen around clip (arrow). Subsequent right mastectomy revealed DCIS measuring 5 mm in vicinity of biopsy site. B D Downloaded from ajronline.org by 90.246.25.176 on 02/27/24 from IP address 90.246.25.176. Copyright ARRS. For personal use only; all rights reserved AJR:194, June 2010 1677 MRI-Occult Breast Cancers median size of the false-negative lesions on final pathology evaluation was 10 mm. There were four false-negative DCIS lesions, one of which was very small by pa[1]thology (5 mm) (Fig. 1). Two other DCIS lesions were obscured by diffusely enhancing surrounding parenchyma (Fig. 2) even at the early phase (75 seconds) of the dynamic study. The remaining multifocal DCIS lesion (grade 3) did not show enhancement in a background of minimal parenchymal enhancement for reasons that are not known. There were three false-negative invasive carcinomas. One 8-mm IDC with extensive DCIS was obscured by diffusely enhancing surround[1]ing parenchyma (Fig. 3). One very small (0.8 mm) IDC with DCIS did not show enhancement in a background of mild parenchymal enhancement for reasons that are not known. The remaining lesion, IDC with DCIS, was visible on T2-weighted images as a low-signal mass; however, no enhancement was noted after the administration of contrast medium. Discussion In this study, DCE-MRI offered a high sensitivity in a cohort of patients with newly diagnosed breast cancer. Sensitivity for all breast carcinoma, invasive carcinoma, and in situ carcinoma was 96.8%, 98.3%, and 90.2%, respec[1]tively. These results are comparable or slightly superior to previously published results [18– 20] (Table 3). This improvement could be explained by the more modern technical parameters used compared with those of prior studies—that is, higher field strength (1.5 T), higher temporal resolution (75 seconds), and higher spatial resolution (slice thickness, 2 mm; in-plane resolution, 1–1.5 mm). The percentage of palpable lesions in this study (84/164 lesions, 51%), when known, was similar to or lower than the percentage of palpable findings in previously reported studies [7, 20]. However, there are some differences in study methodology when this experience is compared with prior reports [18, 20]. Entry criteria in these prior studies include patients with histologically proven benign lesions and those with malignant lesions. In contrast, the reported sensitivity in this study and in the study by Ghaiet al. [19], both retrospective studies, was achieved in cases in which readers knew malignancy was present before reading MRI studies. In the study by Schnall et al. [20], 13 (3.1%) of 422 invasive lesions and 12 (15.6%) of 77 DCIS lesions did not exhibit enhancement at MRI. Teifke et al. [18] found that 28 (8.4%) of 334 invasive lesions and 13 (65%) of 20 DCIS lesions were missed at MRI. In addition, unlike the cases presented in this report, the false-negative lesions described by Teifke et al. included malignancies that were not detected because of technical reasons. No technically inadequate examinations compromised this study and none were included in that of Ghai et al. Despite these differences in study design, the results of this study and all other studies show the existence of MRI-occult breast malignancies. In this study, seven cancers (3.2%) showed no enhancement on MRI. Of the seven false[1]negative cases, four malignancies represented pure DCIS. Pure DCIS lesions often present as nonmass, clumped enhancement in a segmental or linear distribution [23] with plateau or washout curve types [22–25] but with lower peak enhancement values. Non-mass-like enhancement may be more difficult to perceive TABLE 3: Data From Published Articles Compared With Data From This Study Journal, Year of Publication First Author [Reference No.] Total No. of Lesions No. of False-Negative Lesions Sensitivity (%) Total Invasive Cancer In Situ Cancer Total Invasive Cancer In Situ Cancer AJR, 2005 Ghai [19] 104 9 9 — 91.3 91.3 — Radiology, 2002 Teifke [18] 354 41 28 13 88.4 91.6 35.0 Radiology, 2006 Schnall [20] 995 25 13 12 95.0 96.9 84.4 This study 220 7 4 3 96.8 98.3 90.2 Note—Dash (—) indicates no ductal carcinoma in situ present in this population. A Fig. 2—43-year-old woman with screening-detected pleomorphic calcifications measuring 2.5 cm in right breast (case 2 in Table 2). Stereotactic core biopsy revealed ductal carcinoma in situ (DCIS), grade 3. A, Craniocaudal mammographic view of right breast obtained after stereotactic core biopsy shows residual calcifications (arrows). B, On early phase of dynamic contrast-enhanced study (subtraction, T1-weighted images), no abnormal enhancement is observed at area of susceptibility artifact from metallic clip placed after stereotactic core biopsy (arrow) because of diffuse parenchymal enhancement. Needle localization lumpectomy revealed DCIS, grade 3, measuring 3 cm. B Downloaded from ajronline.org by 90.246.25.176 on 02/27/24 from IP address 90.246.25.176. Copyright ARRS. For personal use only; all rights reserved 1678 AJR:194, June 2010 Shimauchi et al. especially in the presence of enhancing breast parenchyma. This observation is supported by a report from the MRI Lexicon Committee indicating that reader agreement for the classification of linear enhancement distribution was poor [24]. There were three cases (two DCIS and one IDC with DCIS) in patients who were younger than the median age of the patient cohort with diffusely enhancing surrounding parenchyma, that could obscure an abnormal enhancing lesion. The median age of these three patients was 46 years, whereas the median age of newly diagnosed cancer patients overall was 58 years. One limitation of this study is that we did not take the hormonal status of our patients into account for scheduling the examinations. At our institution, scheduling patients for breast MRI to stage newly diagnosed cancer has not routinely been done according to the phase in the menstrual cycle for premenopausal women. Perhaps imaging in the second week or at least in the middle of the menstrual cycle, as advocated by Viehweg et al. [26] and Müller-Schimpfle et al. [27], might reduce the number of cases with false-negative MRI due to diffusely enhancing surrounding parenchyma. However, this type of scheduling can be psychologically stressful for patients and can present a perceived delay in care. Of the remaining false-negative malignancies, one was a small (0.8 mm) IDC with DCIS. Teifke et al. [18] suggested that infiltrating cancers associated with DCIS might be difficult to detect on MRI. The sensitivity of MRI for DCIS reported by Teifke et al. was 35%, and those investigators concluded that MRI cannot reliably diagnose DCIS-positive cancers. Although there are some differences in the study methodologies in this study, the sensitivity for DCIS was 90.2%, which suggests that improvement in DCE-MRI technique may aid in achieving a higher sensitivity for DCIS. Further studies are required to prove a definitive association between false-negative MRI examinations and DCIS lesions. Other invasive cancers may not be visualized readily because of very small size or because of diffuse pattern of spread. Prior studies have shown ILC to be MRI occult because of diffuse infiltration without mass formation [16, 17, 19]. However, in this study, all ILC lesions (8.6% of total malignancies) were detected at MRI. The relatively thicker slice thickness (range, 3–10 mm) used in pri[1]or reported studies could have negatively impacted the sensitivity of MRI for ILC. A limitation of this study includes a possible bias because it is a retrospective review. At the time of the retrospective read for this study, re[1]viewers were aware of both the initial and the final histologic findings. In routine practice, we may not be aware of all of the details concerning the histology for all cases, although we routinely have histology or cytology findings for most staging MRI cases at the time of the prospective read. However, this study has shown that improved technical parameters— that is, a higher field strength (1.5 T), higher temporal resolution, higher spatial resolution, and improved coil design—led to better results for the detection of small malignant lesions, particularly DCIS. To summarize, in a population of 220 sequentially diagnosed known breast cancer lesions, we found seven (3.2%) MRI-occult cancers, which is fewer than reported in other published studies. Small tumor size and dif[1]fuse parenchymal enhancement were likely the principal reasons for these false-negative results. Although the overall sensitivity of breast MRI for cancer detection was high (96.8%), it should be emphasized that a negative MRI should not influence the management of a lesion that appears to be of concern on physical examination, mammography, or ultra[1]sound. MRI is complementary to—but is not a replacement for—other breast imaging techniques and should not be used as the sole imag[1]ing study because, as this study shows, a small number of cancers may not be visible at MRI. References 1. Harms SE, Flamig DP, Hesley KL, et al. MR imaging of the breast with rotating delivery of excitation off resonance: clinical experience with pathologic correlation. Radiology 1993; 187:493–501 2. Gilles R, Guinebretiere JM, Lucidarme O, et al. Nonpalpable breast tumors: diagnosis with contrast-enhanced subtraction dynamic MR imaging. Radiology 1994; 191:625–631 3. Kaiser WA. False-positive results in dynamic MR A Fig. 3—49-year-old woman with diffuse pleomorphic and linear calcifications measuring 6.5 cm in right breast, proven to be DCIS, grade 2, by stereotactic core biopsy (case 6 in Table 2). A, Mediolateral magnification view of right breast obtained after stereotactic core biopsy shows calcifications (arrows). Metallic clip (arrowhead) was placed in upper inner quadrant. B, On early phase of dynamic contrast-enhanced study (subtraction, T1-weighted images), no abnormal enhancement is observed in upper outer quadrant of right breast because of diffuse parenchymal enhancement. Right simple mastectomy revealed 8-mm invasive ductal carcinoma, grade 2, with extensive ductal carcinoma in situ. B Downloaded from ajronline.org by 90.246.25.176 on 02/27/24 from IP address 90.246.25.176. Copyright ARRS. For personal use only; all rights reserved AJR:194, June 2010 1679 MRI-Occult Breast Cancers mammography: causes, frequency, and methods to avoid. Magn Reson Imaging Clin N Am 1994; 2:539–555 4. Fobben ES, Rubin CZ, Kalisher L, Dembner AG, Selt[1]zer MH, Santoro EJ. Breast MR imaging with commercially available techniques: radiologic–pathologic correlation. Radiology 1995; 196:143–152 5. Bone B, Aspelin P, Bronge L, Isberg B, Perbeck L, Veress B. Sensitivity and specificity of MR mam[1]mography with histopathological correlation in 250 breasts. Acta Radiol 1996; 37:208–213 6. Liberman L, Morris EA, Dershaw DD, Abramson AF, Tan LK. MR imaging of the ipsilateral breast in women with percutaneously proven breast can[1]cer. AJR 2003; 180:901–910 7. Berg WA, Gutierrez L, NessAiver MS, et al. Diag[1]nostic accuracy of mammography, clinical exami[1]nation, US, and MR imaging in preoperative as[1]sessment of breast cancer. Radiology 2004; 233:830–849 8. Sardanelli F, Giuseppetti GM, Panizza P, et al. Sensitivity of MRI versus mammography for detecting foci of multifocal, multicentric breast cancer in fatty and dense breasts using the whole[1]breast pathologic examination as a gold standard. AJR 2004; 183:1149–1157 9. Schnall MD, Blume J, Bluemke DA, et al. MRI detection of distinct incidental cancer in women with primary breast cancer studied in IBMC 6883. J Surg Oncol 2005; 92:32–38 10. Hollingsworth AB, Stough RG. Preoperative breast MRI for locoregional staging. J Okla State Med Assoc 2006; 99:505–515 11. Bilimoria KY, Cambic A, Hansen NM, Bethke KP. Evaluating the impact of preoperative breast magnetic resonance imaging on the surgical management of newly diagnosed breast cancers. Arch Surg 2007; 142:441–445; discussion, 445–447 12. Lehman CD, Gatsonis C, Kuhl CK, et al. MRI evaluation of the contralateral breast in women with recently diagnosed breast cancer. N Engl J Med 2007; 356:1295–1303 13. Van Goethem M, Schelfout K, Kersschot E, et al. MR mammography is useful in the preoperative locoregional staging of breast carcinomas with extensive intraductal component. Eur J Radiol 2007; 62:273–282 14. Braun M, Polcher M, Schrading S, et al. Influence of preoperative MRI on the surgical management of patients with operable breast cancer. Breast Cancer Res Treat 2008; 111:179–187 15. Kaiser WA. MR mammography [in German]. Ra[1]diologe 1993; 33:292–299 16. Boetes C, Strijk SP, Holland R, Barentsz JO, Van Der Sluis RF, Ruijs JH. False-negative MR imag[1]ing of malignant breast tumors. Eur Radiol 1997; 7:1231–1234 17. Wurdinger S, Kamprath S, Eschrich D, Schneider A, Kaiser WA. False-negative findings of malig[1]nant breast lesions on preoperative magnetic reso[1]nance mammography. Breast 2001; 10:131–139 18. Teifke A, Hlawatsch A, Beier T, et al. Undetected malignancies of the breast: dynamic contrast-en[1]hanced MR imaging at 1.0 T. Radiology 2002; 224:881–888 19. Ghai S, Muradali D, Bukhanov K, Kulkarni S. Nonenhancing breast malignancies on MRI: sonographic and pathologic correlation. AJR 2005; 185:481–487 20. Schnall MD, Blume J, Bluemke DA, et al. Diag[1]nostic architectural and dynamic features at breast MR imaging: multicenter study. Radiology 2006; 238:42–53 21. Van Goethem M, Schelfout K, Kersschot E, et al. Comparison of MRI features of different grades of DCIS and invasive carcinoma of the breast. JBR-BTR 2005; 88:225–232 22. Jansen SA, Newstead GM, Abe H, Shimauchi A, Schmidt RA, Karczmar GS. Pure ductal carcino[1]ma in situ: kinetic and morphologic MR charac[1]teristics compared with mammographic appear[1]ance and nuclear grade. Radiology 2007; 245:684–691 23. Shiraishi A, Kurosaki Y, Maehara T, Suzuki M, Kurosumi M. Extension of ductal carcinoma in situ: histopathological association with MR imag[1]ing and mammography. Magn Reson Med Sci 2003; 2:159–163 24. Ikeda DM, Hylton NM, Kinkel K, et al. Develop[1]ment, standardization, and testing of a lexicon for reporting contrast-enhanced breast magnetic res[1]onance imaging studies. J Magn Reson Imaging 2001; 13:889–895 25. Menell JH, Morris EA, Dershaw DD, Abramson AF, Brogi E, Liberman L. Determination of the presence and extent of pure ductal carcinoma in situ by mammography and magnetic resonance imaging. Breast J 2005; 11:382–390 26. Viehweg P, Paprosch I, Strassinopoulou M, Hey[1]wang-Köbrunner SH. Contrast-enhanced magnetic resonance imaging of the breast: interpreta[1]tion guidelines. Top Magn Reson Imaging 1998; 9:17–43 27. Müller-Schimpfle M, Ohmenhaüser K, Stoll P, Dietz K, Claussen CD. Menstrual cycle and age: influence on parenchymal contrast medium enhancement in MR imaging of the breast. Radiology 1997; 203:145–
Evaluation between 3.0 T vs 1.5 T MRI in Detection of Brain Metastasis using Double Dose Gd-DTPA
Woo-Suk Chung, M.D.1
, Tae-Sub Chung, M.D.1
, Hyung Jung Kim, M.D.2
, Chul Min Ahn,
M.D.2
, Jae Hoon Lee, M.D.1
, Jin Hur, M.D.1
, Arthur Eung-Hyuck Cho, M.D.1
Purpose : Early detection of small brain metastases is important. The purpose of this
study was to compare the detectability of brain metastases according to the size
between 1.5 T and 3.0 T MRI.
Materials and Methods : We reviewed 162 patients with primary lung cancer who
were examined for TNM staging. After administration of double dose of Gd-DTPA,
MR imaging was performed with SPGR by 3.0 T MRI and then with T1 SE sequence
by 1.5 T MRI. In each patient, three readers performed qualitative assessment.
Sensitivity, positive predictive value, and diagnostic accuracy were calculated in 3.0
T and 1.5 T MRI according to size. Using the signal intensity (SI) measurements
between the metastatic nodules and adjacent tissue, nodule-to-adjacent tissue SI ratio
was calculated.
Results : Thirty-one of 162 patients had apparent metastatic nodules in the brain at
either 1.5 T or 3.0 T MR imaging. 143 nodules were detected in 3.0 T MRI, whereas
137 nodules were detected at 1.5 T MRI. Six nodules, only detected in 3.0 T MRI,
were smaller than 3.0 mm in dimension. Sensitivity, positive predictive value, and
diagnostic accuracy in 3.0 T MRI were 100 %, 100 %, and 100 % respectively, and
in 1.5 T MRI were 95.8 %, 88.3 %, and 85.1 % respectively. SI ratio was significantly
higher in the 3.0 T MRI than 1.5 T MRI (p=0.025).
Conclusion : True positive rate of 3.0 T MRI with Gd-DTPA was superior to 1.5 T
MRI with Gd-DTPA in detection of metastatic nodules smaller than 3.0 mm.
Index words :Magnetic resonance (MR), high-field-strength imaging
Brain neoplasm, metastases
Contrast medium
Introduction
Brain metastases present a poor prognosis suggesting a shortened survival time. Early diagnosis of brain
involvement and determination of the number of metastases are important not only for quality of life but
also for cost effectiveness (1-3). The decision regarding a conservative versus a surgical approach depends on the number of brain metastases detected by radiologic means (1-3).
A contrast-enhanced MRI has become the method of choice for visualization of brain metastases (1). Highdose gadolinium-enhanced MR examinations may have advantages over 0.1 mmol/kg examinations in detecting early and/or small metastases (3-7). A delayed study can also increase the contrast (2). Comparing to the 1.5 T MRI with an axial T1-weighted spin echo sequence (SE), the higher field strength MR systems combined with a sequence of SPGR (spoiled gradient recalled acquisition in the steady state) and administration of a high dose of Gd-DTPA (gadopentetate dimeglumine) may also have advantages in detecting small metastases, although this has not yet been verified by clinical data. The purpose of this study is to compare the detectability of brain metastases classified according to the size of nodules, between thin slice SPGR of 3.0 T and conventional thick slice SE of 1.5 T MRI with the administration of a double dose of GdDTPA. Materials and Methods
Patients From December 2002 to February 2004, a total of 162
consecutive patients with primary lung cancer
participated in our study. The institutional review board approved our study, and informed consent was
obtained from all patients regarding the potential risks of both the double dose of contrast medium and two assessments by MRI scanning on a 3.0 T and 1.5 T machine. After the study, 31 patients of this population were diagnosed as having brain metastases. Mean patient age was 61.2 (range, 43 to 80 years).
Protocol
On the 3.0 T MR scanner (GE Signa VH/i; GE
medical system, Milwaukee, USA), the images were acquired using a standard head coil and an actively
shielded gradient system with a maximum gradient strength of 43 mT/m. On the 1.5 T MR scanner
(Magnetom Vision; Siemens Medical Systems, Erlangen, Germany), a standard head coil and a
maximum gradient strength of 25 mT/m were used.
All patients were examined after administrating a contrast agent with a double dose of Gd-DTPA (0.2
mmol/kg). First, examinations were performed on a 3.0 T MR scanner and then were subsequently performed on a 1.5 T MR scanner without additional contrast injection. The scan interval between the 3.0 T and 1.5 T MR examination was less than 20 minutes. The contrast agent used was Gd-DTPA (Magnevist;
Schering AG, Berlin, Germany). In all patients, the double dose of Gd-DTPA at 0.2 mmol/kg was
administered intravenously as a bolus and scanning commenced immediately.
MR imaging included the following sequences on both scanners: At 3.0 T MRI, an axial 3D SPGR, which
is usually used at present, was used (TR/TE/TI = 5.7/1.44/400 milliseconds; flip angle 20°; 2 mm slice
thickness; FOV of 220 mm; matrix size of 512×512 ZIP; spatial resolution of 0.43×0.43×2 mm; two
acquisition) with a scan time of 3 minutes 30 seconds. At 1.5 T MRI, an axial T1-weighted spin echo sequence was used (TR/RE = 600/14 milliseconds; flip angle 90°; 5 mm slice thickness; FOV of 210 mm; matrix size of 174×256; spatial resolution of 1.2×0.82×5 mm; two acquisition) with a scan time of 5 minutes 30 seconds.
Three radiologists performed randomized, independent blinded review. The three readers were
merely informed that all of the patients had lung malignancies. The postcontrast MR examinations of the
brain on all of the patients were evaluated. The readers did not have access to other image sets within each study.
The presence, size, and number of metastatic nodules were assessed. The postcontrast images were divided into the following two groups: 3.0 T MRI with a double dose of Gd-DTPA and 1.5 T MRI with a double dose of Gd-DTPA. The readers were asked to document the number of nodules. Each nodule in each study was numbered and classified according to its largest diameter measurement: ≤3 mm, 3 mm to 5 mm, or 5 mm.
Woo-Suk Chung et al
- 102 –
Subsequently, if there was any debate about nodule
classification, a final interpretation using imaging
studies was done until consensus among the readers
was accomplished. If disagreements persisted, 3-6
months of follow-up MR scans were obtained for
further validation. For the patients who had follow-up
MR scans, these scans were considered positive for
metastases if there was a response to the treatment or
if there was a growth of nodules identified during the
follow-up period.
In addition, the sensitivity, specificity, positive
predictive value, negative predictive value, and
diagnostic accuracy of the 3.0 T and 1.5 T MRI were
calculated using the results from the final interpretation
according to size.
A quantitative image assessment was performed next.
The same lesions on scans of the two different MRI
systems were determined by comparing peripheral
structures. Signal intensities of adjacent tissue and
nodule were assessed by region of interest (ROI)
measurements placed identically on both series of
images using the same sized circular ROI from the
software available on both scanners. Nodule-toadjacent tissue contrast (SI ratio) is defined by
SI ratio = ×100
Stissue1 taken as the signal intensity of an ROI assessed
over a nodule and Stissue2 taken as the signal intensity
assessed over the contralateral white matter. The signal
intensity of a nodule was measured within an
enhanced area. In inhomogeneously enhanced nodules,
the area of maximum uptake was chosen for
measurement.
Statistical Analysis
The paired Wilcoxon’s signed ranks test was used to
compare qualitative scores, and the matched-pair-t test
was used to compare nodule SI ratios between the 3.0
T and 1.5 T MRI with a double dose of Gd-DTPA. For
all tests, significance was set at p < 0.05, and SPSS software (SPSS Inc. Chicago, US) was used for statistical analyses. Results In the final review made by consensus of three radiologists (Table 1), a total of 143 metastatic nodules were detected by the 3.0 T MRI with a double dose of Gd-DTPA. Of these nodules, 49 nodules were ≤ 3 mm in diameter, 37 nodules were 3 mm to 5 mm, and 57 nodules were >5 mm. A total of 137 metastatic
nodules were detected by the 1.5 T MRI with a double
dose of Gd-DTPA in the final interpretation. Of these
nodules, 43 nodules were ≤ 3 mm in diameter. The
3.0 T MRI with a double dose of Gd-DTPA was
significantly more effective at detecting nodules smaller
than 3 mm (p = 0.014) than the 1.5 T MRI. However,
we found no significant difference in the detection of
nodules larger than 3 mm (p > 0.05) (Table 1).
In 6 of the 31 cases, six nodules, which were only
detected by the 3.0 T MRI with a double dose of GdDTPA, and missed by the 1.5 T MRI with a double
dose of Gd-DTPA, were unanimously agreed upon by
all participants in the final interpretation. In three of
the six nodules, a growing nodule was detected during
a follow-up MR scan which confirmed the presence of
metastases (Fig. 1). Three of the 6 nodules missed were
unable to be detected using the 1.5 T MRI with a
double dose of Gd-DTPA due to artifact (Fig. 2). Finally
the 3.0 T MRI with a double dose of Gd-DTPA was
Stissue1 – Stissue2
Stissue2
Evaluation between 3.0 T vs 1.5 T MRI in Detection of Brain Metastasis using Double Dose Gd-DTPA- 103 –
Table 1. Total Number of Brain Metastases Classified by Size
and Detected at the Final Interpretation
Size 3.0 T MRI with 1.5 T MRI with p value Gd-DTPA Gd-DPTA
≤ 3 mm 49 43 0.014
3 mm<-≤5 mm 37 37 > 0.05
5 mm< 57 57 > 0.05
Total 143 137 0.014
Note : Data are the number of nodules
Table 2. The Values of Currently Used Statistical Measures for
the 3.0 T and 1.5 T MRIs with Gd-DTPA According to Nodule
Size
Metastatic nodules
Total ≤ 3 mm
Present Absent Present Absent
3.0 T MRI with Positive 143 00* 49 0*
Gd-DTPA Negatiive 000 00* 00 0*
1.5 T MRI with Positive 137 18* 43 18*
Gd-DTPA Negative 006 00* 06 0*- Pseudolesions that were detected as nodules by the 1.5 T MRI
were detected as vascular structures by the 3.0 T MRI.
useful for confirming the presence of these nodules.
Nodules observed by the 3.0 T MRI with a double
dose of Gd-DTPA were brighter or better delineation
than by the 1.5 T MRI with a double dose of Gd-DTPA.
Out of 137 nodules, 65 were brighter (Fig. 3) and out of
137 nodules, 63 were better delineated (Fig. 4). Fortyfive of 137 nodules were satisfactory in both conditions.
By consensus, the final review stated, nodules
detected by the 3.0 T and 1.5 T MRI were considered
positive for metastases (Table 2). Additionally, there
were 18 pseudolesions which were detected as nodules
by the 1.5 T MRI, but as vascular structures by the 3.0
T MRI (Fig. 5). The sensitivity, positive predictive
values, and diagnostic accuracy of the 1.5 T MRI with
a double dose of Gd-DTPA were 95.8%, 88.3%, and
85.1% respectively. The sensitivity, positive predictive
value, and diagnostic accuracy of the 3.0 T MRI with
a double dose of Gd-DTPA were 100%, 100%, and
Woo-Suk Chung et al- 104 –
a b
Fig. 2. A 73-year-old male with multiple brain metastases. Metastatic nodule (a) by a 3.0 T MR image with a double
dose of Gd-DTPA show a metastatic nodule (arrow). But (b) the 1.5 T MR image with a double dose of Gd-DTPA
cannot show the metastatic nodule by artifact.
abc
Fig. 1. A 65-year-old male with single brain metastasis. (a) The 3.0 T MR image with a double dose of Gd-DTPA
shows a small metastatic nodule (arrow). (b) The 1.5 T MR image with a double dose of Gd-DTPA cannot show
the nodule. In (c), a growing nodule was detected during the follow-up MRI with a double dose of Gd-DTPA which
confirmed the presence of metastasis (arrow).
100% respectively. For metastatic nodules smaller than
3 mm, the sensitivity, positive predictive value, and
diagnostic accuracy of the 1.5 T MRI with a double
dose of Gd-DTPA were 87.8%, 70.5%, and 64.2%
respectively. The sensitivity, positive predictive value,
and diagnostic accuracy of 3.0 T MRI with a double
dose of Gd-DTPA were 100%, 100%, and 100%
respectively (Table 3).
For quantitative image assessment, the SI ratio in the
post contrast sequences (Table 4) was significantly
higher in the 3.0 T MRI with a double dose of GdDTPA than in the 1.5 T MRI with a double dose of GdDTPA (p = 0.025).
Discussion
Detection of metastatic nodules is dependent on both
their size and contrast ratio (2). As methods to increase
the contrast, a higher dose of Gd-DTPA, a higher field
strength, and delayed study can all be used to aid
detection (2-5, 8-17). Nodules larger than 10 mm are
easily detected because vasogenic edema is customarily
associated with larger metastases (2). So, when a higher
dose of Gd-DTPA is used, the detection rate of larger
nodules is not influenced. However, a higher dose is
helpful for detecting small nodules because it increases
nodule enhancement, yet this method has the
disadvantages of increasing the false positive rate and
promoting side effects (2-5, 8-14). A delayed study
can also increase the contrast (2). It has been
recommended that image acquisition be delayed from
5 to 35 minutes after the administration of contrast
material at a dose of 0.1 mmol/kg to ensure optimal
detection (2, 15).
Three of the 6 nodules missed were unable to be
detected using the 1.5 T MRI with a double dose of GdDTPA due to partial volume artifact. Eighteen lesions
were detected in the 1.5 T MRI with a double dose of
Gd-DTPA, which proved to be vascular structures in
3.0 T MRI with a double dose of Gd- DTPA. The true
nature of these lesions was revealed because of the
greater morphologic detail visualized by the high field
Evaluation between 3.0 T vs 1.5 T MRI in Detection of Brain Metastasis using Double Dose Gd-DTPA- 105 –
a b
Fig. 3. A 65-year-old male with
multiple brain metastases. (a) The
3.0 T MR image with a double
dose of Gd-DTPA shows a
metastatic nodule with spherical
enhancement (arrow). (b) The 1.5
T MR image with a double dose
of Gd-DTPA shows a metastatic
nodule with spherical enhancement (arrow). Nodules observed
by the 3.0 T MRI with a double
dose of Gd-DTPA were brighter
than when observed by the 1.5 T
MRI with a double dose of GdDTPA.
Table 3. Estimation of the Values of Currently Used Statistical
Measures for the 3.0 T and 1.5 T MRI with Gd-DTPA According
to Nodule Size
Metastatic nodules
Total ≤ 3 mm
3.0 T 1.5T 3.0 T 1.5T
Sensitivity 100 95.8 100 87.8
Specificity – 0 – 0
Positive predictive value 100 88.3 100 70.5
Negative predictive value – 0 – 0
Diagnostic accuracy 100 85.1 100 64.2
Table 4. Results of a quantitative assessment of nodules that
were detected by the 3.0 T and 1.5 T MRIs with Gd-DTPA
(n=137)
3.0 T 1.5 T P value
Nodules-to-adjacent tissue: SI ratio 94.59 63.86 0.025
strength MR image, thus allowing differentiation
between true enhancing lesions and sulcal vessels.
In our study, each nodules was classified according
to its largest diameter as being ≤ 3 mm, 3 mm to 5
mm, and >5 mm because the slice thickness of the 3.0
T MRI was 2.0 mm and 5 mm for the 1.5 T MRI.
Therefore this study classified small metastatic nodules
as being smaller than 3 mm.
Although our study supports the use of a higher field
strength MRI with a double dose of Gd-DTPA for
increased metastatic nodule detection and for improved
nodule enhancement and delineation, the results
should be interpretated with caution. The reason for
this is that for patients with two or more brain
metastases, additional metastases found with the 3.0 T
MRI seem to be of limited clinical importance. The
presence of two or more small nodules generally will
not change the way the patient is managed. Therefore,
it is of utmost importance to identify the difference
between none, one, and more than one metastatic
nodule. Patients with a single metastatic nodule located
in a respectable region can be treated surgically, and
the tumor staged as M1, not M0. However, patients
with two or more metastatic nodules are usually
Woo-Suk Chung et al- 106 –
a b
Fig. 5. A 67-year-old female with
multiple brain metastases. (a) The
3.0 T MRI with a double dose of
Gd-DTPA shows vascular
structure (arrow). (b) The 1.5 T
MRI with a double dose of GdDTPA shows a nodular lesion
(arrow). A pseudolesion that was
detected as a nodule by the 1.5 T
MRI with a double dose of GdDTPA but as a vascular structure
by the 3.0 T MRI with a double
dose of Gd-DTPA is showed in
this figure.
a b
Fig. 4. A 64-year-old male with
multiple brain metastases. (a) The
3.0 T MRI with a double dose of
Gd-DTPA shows a metastatic
nodule with ring enhancement
(arrow). (b) The 1.5 T MRI with
Gd-DTPA show a metastatic
nodule with ring enhancement
(arrow). Nodules observed by the
3.0 T MRI with a double dose of
Gd-DTPA were better delineated
than when observed by the 1.5 T
MRI with a double dose of GdDTPA.
treated with radiation therapy and/or systemic
chemotherapy (3, 18-22). On the other hand, the use
of a higher strength field MRI was found to be helpful
in confirming the appearance of an equivocal
metastatic nodule. The 3.0 T MRI with a double dose
of Gd-DTPA was also useful for the detection of
additional metastases in patients with a known lesion
detected by the 1.5 T MRI with a double dose of GdDTPA. Because this also has influence on the
sensitivity, positive predictive value, and diagnostic
accuracy the 3.0 T MRI with a double dose of GdDTPA was found to have better results than the 1.5 T
MRI with a double dose of Gd-DTPA in our study.
Therefore we recommend the use of the 3.0 T MRI
with a double dose of Gd-DTPA in only three
circumstances: when the findings by the 1.5 T MRI
with Gd-DTPA are equivocal, when one potentially
surgically respectable nodule is identified, or for
detecting early and/or small metastases
There were two limitations in this study. The first
limitation was a difference in protocol sequence. The
T1 weighted spin-echo protocol was used for the 1.5 T
MRI and SPGR technique protocol for the 3.0 T MRI.
Detectability of metastatic nodules is more effective
with 3.0 T MRI with T1 SE than with a 1.5 T MRI with
SE (2). SPGR with thin slice thickness is superior to the
T1 spin echo sequence with thick slice thickness due
to a partial volume effect. Additionally, SE is not
optimum for a 3.0 T due to longer T1- and shorter T2-
relaxation times of water protons, which decrease the
contrast ratio in the 3.0 T images.17 The purpose of
this study was to detect early small brain metastases,
so using the SPGR sequence with a 3.0 T MRI, which
is usually used at present, has advantages for detecting
early small brain metastases. The second limitation was
that the scan interval between the 1.5 T and 3.0 T MRI
was less than 20 minutes. However, a delayed study
increases contrast as mentioned above. The current
study found that the SI ratio was significantly higher in
the 3.0T images than in the 1.5 T images. Considering
the delayed study by the 1.5 T MRI, this limitation
emphasizes the better detection rate of the 3.0 T MRI
with a double dose of Gd-DTPA. And due to these
confounding variables, blinded reviews were
performed by three readers.
The detectability of metastatic nodules smaller than
3mm was better using a 3.0 T MRI with SPGR than a
1.5 T MRI with T1 SE. Therefore we recommend a 3.0
T MRI with SPGR and a double dose of Gd-DTPA for
detecting early and/or small metastatic nodules,
furthermore influencing treatment.
References
1.Lassman AB, DeAngelis LM. Brain metastases. Neurol Clin.
2003; 21:1-23, vii, Review
2.Ba-Ssalamah A, Nobauer-Huhmann IM, Pinker K, et al. Effect
of contrast dose and field strength in the magnetic resonance
detection of brain metastases. Invest Radiol 2003; 38: 415-422
3.Sze G, Johnson C, Kawamura Y, et al. Comparison of singleand triple-dose contrast material in the MR screening of brain
metastases. AJNR Am J Neuroradiol 1998; 19:821-828
4.Runge VM, Kirsch JE, Burke VJ, et al. High-dose Gadoteridol
in MR imaging of intracranial neoplasm. J Magn Reson
Imaging 1992; 2:9-18
5.Yuh WT, Engelken JD, Muhonen MG, Mayr NA, Fisher DJ,
Ehrhardt JC. Experience with high-dose gadolinium MR
imaging in the evaluation of brain metastases. AJNR Am J
Neuroradiol 1992; 13:335-345
6.Brekenfeld C, Foert E, Hundt W, Kenn W, Lodeann KP, Gehl
HB. Enhancement of cerebral diseases: How much contrast
agent is enough? Comparison of 0.1, 0.2, and 0.3 mmol/kg
Gadoteridol at 0.2 T with 0.1 mmol/kg Gadoteridol at 1.5 T.
Invest Radiol 2001; 36:266-275
7.Yuh WT, Tali ET, Nguyen HD, Simonson TM, Mayr NA,
Fisher DJ. The effect of contrast dose, imaging time, and
lesion size in the MR detection of intracerebral metastasis.
AJNR Am J Neuroradiol 1998; 16:373-380
8.Van Dijk P, Sijens PE, Schmitz PI, Oudkerk M. Gd-enhanced
MR imaging of brain metastases: contrast as a function of
dose and lesion size. Magn Reson Imaging 1997; 15:535-541
9.Yuh WT, Fisher DJ, Engelken JD, et al. MR evaluation of
CNS tumors: dose comparison study with gadopentetate
dimeglumine and gadoteridol. Radiology 1991; 180:485-491
10.Healy ME, Hasselink JR, Press GA, Middleton MS. Increased
detection of intracranial metastases with intravenous GdDTPA. Radiology 1987; 165:619-624
11.Akeson P, Vikhoff B, Stahlberg F, Holtas S. Brain lesion
contrast in MR imaging: dependence of field strength and
concentration of gadodiamide injection in patients and
phantoms. Acta Radiol 1977; 38:14-18
12.Runge VM. A review of contrast media research in 1999 –
- Invest Radiol 2001; 36:123-130
13.Yuh WT, Parker JR Carvlin MJ. Indication related dosing for
magnetic resonance contrast media. Eur Radiol 1977; 7(suppl
5):S269-S275
14.Rinck PA, Muller RN. Field strength and dose dependence of
contrast enhancement by Gadolinium-based MR contrast
agents. Eur Radiol 1999; 9:998-1004
15.Schorner W, Laniado M, Niendorf HP, Schubert C, Felix R.
Time-dependent changes in image contrast in brain tumors
after gadolinium-DTPA. AJNR Am J Neuroradiol 1986;
Evaluation between 3.0 T vs 1.5 T MRI in Detection of Brain Metastasis using Double Dose Gd-DTPA
- 107 –
7:1013-1020
16.Chang KH, Ra DG, Han MH, Cha SH, Kim HD, Han MC.
Contrast enhancement of brain tumors at different MR field
strengths: comparison of 0.5 T and 2.0 T. AJNR Am J
Neuroradiol 1994; 15:1413-1419
17.Nobauer-Huhmann IM, Ba-Ssalamah A, Mlynarik V, et al.
Magnetic resonance imaging contrast enhancement of brain
tumors at 3 tesla versus 1.5 tesla. Invest Radiol 2002; 37:114-
119
18.Galicich JH, Sundaresan N, Thaler HT. Surgical treatment of
single brain metastasis: evaluation of results by computerized
tomography scanning. J Neurosurg 1980; 53:63-67
19.Runge VM, Carollo BR, Wolf CR, Nelson KL, Gelblum DY.
Gd-DTPA: A review of clinical indications in central nervous
system magnetic resonance imaging. Radiographics 1989;
9:929-958
20.Earnest F 4th, Ryu JH, Miller GM, et al. Suspected non-small
cell lung cancer: Incidence of occult brain and skeletal
metastases and effectiveness of imaging for detection-Pilot
study. Radiology 1999; 211:137-145
21.Koutras AK, Marangos M, Kourelis T, et al. Surgical
management of cerebral metastases from non-small cell lung
cancer. Tumori 2003; 89:292-297
22.Soffietti R, Ruda R, Mutani R. Management of brain
metastases. J Neurol 2002; 249:1357-1369. Review
Woo-Suk Chung et al- 108 –
통신저자 : 정태섭, 서울특별시 강남구 도곡동 146-92 연세대학교 영동세브란스병원 영상의학과
Tel. 82-2019-3514 Fax. 82-3462-5472 E-mail: tschung@yumc.yonsei.ac.kr
뇌전이종양의발견에있어서Doble dose Gd-DTPA를이용한
3 T MRI와1.5 T MRI간의비교연구
1
연세대학교 영동세브란스병원 영상의학과
2
연세대학교 영동세브란스병원 호흡기내과
정우석1
·정태섭1
·김형중2
·안철민2
·이재훈1
·허 진1
·조응혁1
목적: 작은 뇌전이 종양의 조기 발견은 중요하다. 이 연구의 목적은 1.5 T MRI와 3.0 T MRI 간의 크기에 따른 뇌
전이 종양의 발견율을 비교하는 것이다.
대상 및 방법: 폐암으로 진단 받은 162명의 환자를 대상으로 TNM 병기를 위해 뇌 MRI를 시행하였다. Gd-DTPA
를 2배 용량으로 투여 후, 3.0 T MRI에서 훼손경사회복획득으로 촬영하였으며 그 후 1.5 T MRI에서 T1 스핀 에
코로 촬영하였다. 3명의 방사선과 전문의가 합의하여 MRI를 판독하였으며 정성 평가를 시행하였다. 3.0 T와 1.5 T
MRI에서 크기에 따라 민감도, 양성 예측률, 정확도를 평가하였다. 신호 강도를 사용하여 전이 종양과 인접 조직간
의 신호강도 비를 계산하였다.
결과: 162명의 환자 중 31명에서 1.5 T 또는 3.0 T MR에 뇌전이 종양이 발견되었다. 3.0 T MRI에서 143개의 종
양이 발견되었으나 1.5 T MRI에서 137개의 종양이 발견되었다. 6개의 종양이 3.0 T MRI에서만 발견되었으며 크
기는 모두 3 mm 미만이었다. 3.0 T MRI의 민감도, 양성 예측률, 정확도는 각각 100%, 100%, 100%이며, 1.5 T
MRI에서는 각각 95.8%, 88.3%, 85.1% 이다. SI ratio는 1.5 T MRI보다 3.0 T MRI에서 유의하게 높았다
(p=0.025).
결론: Double dose Gd-DTPA를 이용한 3.0 T MRI는 3 mm미만의 뇌전이 종양을 발견하는데 있어서 1.5 T MRI
보다 우수하다.
4/16/20
Malcolm’s brain MRI was initially read as completely normal. Upon getting a second opinion, it was discovered that 22 lesions suggestive of multiple sclerosis had been missed. Here is his story.
[Malcolm]
After 18 months of deteriorating health, with a multitude of neurological symptoms, I went to see a neurologist. I was ordered a standard brain MRI to help figure out what was going on. By this time, I had been researching my symptoms for a few months, and, in doing so, had developed a basic understanding of what I was looking for: T2 lesions.
When I received the results from my MRI, the report said everything was normal. I met with my neurologist, and he too said there was nothing abnormal about my scan. When I viewed the MRI myself, however, I found 4 spots that looked to me to be brain lesions. I brought them up to my doctor at my next appointment but was essentially laughed at.
It was quite unsettling to have something so concerning be shrugged off. After that appointment, I decided I wanted to switch to a new neurologist and get a second opinion on my MRI scan. I made an appointment with the new doctor, but it was going to be another 6 weeks before I could be seen. Fortunately, I found DocPanel. I submitted my MRI for review and, within 48 hours, I got my results back.
[Dr. Michael Rozenfeld]
Malcolm reached out to DocPanel due to concern for a missed diagnosis. He was reportedly having symptoms suspicious for multiple sclerosis but his MRI was initially read as completely normal. Based on my review of his MRI, the exam was not normal. Malcolm had significant findings that were certainly suggestive of, but not entirely specific for, multiple sclerosis.
[Malcolm]
My second opinion results were quite alarming – there were not 4 lesions, but 22. Twenty-two! They were small, but they were most definitely there. I took the second opinion report to my new neurologist, and now I am being considered for possible Primary Progressive Multiple Sclerosis. I am scheduled to get new MRIs in November to confirm or rule out MS and you can be sure I will be sending them to DocPanel for a second look!
I have found that the most important thing people in my shoes can do is be your own advocate. MS is such an odd and individual disease. – Malcolm
[Dr. Michael Rozenfeld]
Radiology exams can be misinterpreted for a number of reasons – this is unfortunately not uncommon. While MRI is not the only piece in the puzzle for MS diagnosis, it plays a significant role. A false negative diagnosis made off an MRI scan could lead the neurologist and patient down an incorrect path and delay an accurate diagnosis, or potentially miss it entirely.
While MRI is not the only piece in the puzzle for MS diagnosis, it plays a significant role. A false negative diagnosis made off an MRI scan could lead the neurologist and patient down an incorrect path and delay an accurate diagnosis, or potentially miss it entirely.
The positive findings on Malcolm’s exam and his new report provide additional essential information for his neurologist, allowing him (the neurologist) to combine these imaging findings with other test results and physical exam findings to come to an accurate diagnosis.
I would always suggest patients seek a second opinion for complex medical diagnoses, especially if the patient feels that something is being missed or that their concerns aren’t being addressed. This could mean setting up an appointment with a second neurologist or submitting your radiology exams for a second opinion to a company such as DocPanel, where they will be interpreted by subspecialty trained and certified neuroradiologists. I personally like to explain the imaging findings in layman’s terms, to ensure that the patient understands the disease process. I believe this empowers patients so that they can take an active role in ensuring the best decisions regarding their care are made.
[Malcolm]
After a few months of working with my current neurologist and a support group of people diagnosed with MS, I have found that the most important thing people in my shoes can do is be your own advocate. MS is such an odd, and individual disease. No two patients are exactly alike in their presentation or their symptoms. This can make it very difficult to diagnose. It is always helpful to get several opinions; your health is just to darn important.
DocPanel is committed to making sure every patient receives excellent care. If you would like an expert second opinion on your medical imaging scan from Dr.Rozenfeld or one of our other neuroradiology subspecialists, you can learn more here.

Evaluation between 3.0 T vs 1.5 T MRI in Detection of Brain Metastasis using Double Dose Gd-DTPA Woo-Suk Chung, M.D.1 , Tae-Sub Chung, M.D.1 , Hyung Jung Kim, M.D.2 , Chul Min Ahn, M.D.2 , Jae Hoon Lee, M.D.1 , Jin Hur, M.D.1 , Arthur Eung-Hyuck Cho, M.D.1 Purpose : Early detection of small brain metastases is important.
The purpose of this study was to compare the detectability of brain metastases according to the size between 1.5 T and 3.0 T MRI. Materials and Methods :
We reviewed 162 patients with primary lung cancer who were examined for TNM staging. After administration of double dose of Gd-DTPA, MR imaging was performed with SPGR by 3.0 T MRI and then with T1 SE sequence by 1.5 T MRI. In each patient, three readers performed qualitative assessment. Sensitivity, positive predictive value, and diagnostic accuracy were calculated in 3.0 T and 1.5 T MRI according to size. Using the signal intensity (SI) measurements between the metastatic nodules and adjacent tissue, nodule-to-adjacent tissue SI ratio was calculated. Results : Thirty-one of 162 patients had apparent metastatic nodules in the brain at either 1.5 T or 3.0 T MR imaging. 143 nodules were detected in 3.0 T MRI, whereas 137 nodules were detected at 1.5 T MRI. Six nodules, only detected in 3.0 T MRI, were smaller than 3.0 mm in dimension. Sensitivity, positive predictive value, and diagnostic accuracy in 3.0 T MRI were 100 %, 100 %, and 100 % respectively, and in 1.5 T MRI were 95.8 %, 88.3 %, and 85.1 % respectively. SI ratio was significantly higher in the 3.0 T MRI than 1.5 T MRI (p=0.025). Conclusion : True positive rate of 3.0 T MRI with Gd-DTPA was superior to 1.5 T MRI with Gd-DTPA in detection of metastatic nodules smaller than 3.0 mm. Index words :Magnetic resonance (MR), high-field-strength imaging Brain neoplasm, metastases Contrast medium Introduction Brain metastases present a poor prognosis suggesting a shortened survival time. Early diagnosis of brain involvement and determination of the number of metastases are important not only for quality of life but also for cost effectiveness (1-3).
The decision regarding a conservative versus a surgical approach depends on the number of brain metastases detected by radiologic means (1-3). A contrast-enhanced MRI has become the method of choice for visualization of brain metastases (1). High[1]dose gadolinium-enhanced MR examinations may have advantages over 0.1 mmol/kg examinations in detecting early and/or small metastases (3-7).
A delayed study can also increase the contrast (2). Comparing to the 1.5 T MRI with an axial T1-weighted spin echo sequence (SE), the higher field strength MR systems combined with a sequence of SPGR (spoiled gradient recalled acquisition in the steady state) and administration of a high dose of Gd-DTPA (gadopentetate dimeglumine) may also have advantages in detecting small metastases, although this has not yet been verified by clinical data. The purpose of this study is to compare the detectability of brain metastases classified according to the size of nodules, between thin slice SPGR of 3.0 T and conventional thick slice SE of 1.5 T MRI with the administration of a double dose of Gd[1]DTPA.
Materials and Methods Patients From December 2002 to February 2004, a total of 162 consecutive patients with primary lung cancer participated in our study.
The institutional review board approved our study, and informed consent was obtained from all patients regarding the potential risks of both the double dose of contrast medium and two assessments by MRI scanning on a 3.0 T and 1.5 T machine. After the study, 31 patients of this population were diagnosed as having brain metastases. Mean patient age was 61.2 (range, 43 to 80 years). Protocol On the 3.0 T MR scanner (GE Signa VH/i; GE medical system, Milwaukee, USA), the images were acquired using a standard head coil and an actively shielded gradient system with a maximum gradient strength of 43 mT/m. On the 1.5 T MR scanner (Magnetom Vision; Siemens Medical Systems, Erlangen, Germany), a standard head coil and a maximum gradient strength of 25 mT/m were used. All patients were examined after administrating a contrast agent with a double dose of Gd-DTPA (0.2 mmol/kg). First, examinations were performed on a 3.0 T MR scanner and then were subsequently performed on a 1.5 T MR scanner without additional contrast injection.
The scan interval between the 3.0 T and 1.5 T MR examination was less than 20 minutes. The contrast agent used was Gd-DTPA (Magnevist; Schering AG, Berlin, Germany). In all patients, the double dose of Gd-DTPA at 0.2 mmol/kg was administered intravenously as a bolus and scanning commenced immediately. MR imaging included the following sequences on both scanners:
At 3.0 T MRI, an axial 3D SPGR, which is usually used at present, was used (TR/TE/TI = 5.7/1.44/400 milliseconds; flip angle 20°; 2 mm slice thickness; FOV of 220 mm; matrix size of 512×512 ZIP; spatial resolution of 0.43×0.43×2 mm; two acquisition) with a scan time of 3 minutes 30 seconds. At 1.5 T MRI, an axial T1-weighted spin echo sequence was used (TR/RE = 600/14 milliseconds; flip angle 90°; 5 mm slice thickness; FOV of 210 mm; matrix size of 174×256; spatial resolution of 1.2×0.82×5 mm; two acquisition) with a scan time of 5 minutes 30 seconds.
Three radiologists performed randomized, independent blinded review. The three readers were merely informed that all of the patients had lung malignancies. The postcontrast MR examinations of the brain on all of the patients were evaluated. The readers did not have access to other image sets within each study. The presence, size, and number of metastatic nodules were assessed. The postcontrast images were divided into the following two groups: 3.0 T MRI with a double dose of Gd-DTPA and 1.5 T MRI with a double dose of Gd-DTPA. The readers were asked to document the number of nodules. Each nodule in each study was numbered and classified according to its largest diameter measurement: ≤3 mm, 3 mm to 5 mm, or >5 mm. Woo-Suk Chung et al – 102 – Subsequently, if there was any debate about nodule classification, a final interpretation using imaging studies was done until consensus among the readers was accomplished. If disagreements persisted, 3-6 months of follow-up MR scans were obtained for further validation.
For the patients who had follow-up MR scans, these scans were considered positive for metastases if there was a response to the treatment or if there was a growth of nodules identified during the follow-up period. In addition, the sensitivity, specificity, positive predictive value, negative predictive value, and diagnostic accuracy of the 3.0 T and 1.5 T MRI were calculated using the results from the final interpretation according to size. A quantitative image assessment was performed next. The same lesions on scans of the two different MRI systems were determined by comparing peripheral structures. Signal intensities of adjacent tissue and nodule were assessed by region of interest (ROI) measurements placed identically on both series of images using the same sized circular ROI from the software available on both scanners. Nodule-to[1]adjacent tissue contrast (SI ratio) is defined by SI ratio = ×100 Stissue1 taken as the signal intensity of an ROI assessed over a nodule and Stissue2 taken as the signal intensity assessed over the contralateral white matter.
The signal intensity of a nodule was measured within an enhanced area. In inhomogeneously enhanced nodules, the area of maximum uptake was chosen for measurement. Statistical Analysis The paired Wilcoxon’s signed ranks test was used to compare qualitative scores, and the matched-pair-t test was used to compare nodule SI ratios between the 3.0 T and 1.5 T MRI with a double dose of Gd-DTPA. For all tests, significance was set at p < 0.05, and SPSS software (SPSS Inc. Chicago, US) was used for statistical analyses. Results In the final review made by consensus of three radiologists (Table 1), a total of 143 metastatic nodules were detected by the 3.0 T MRI with a double dose of Gd-DTPA. Of these nodules, 49 nodules were ≤ 3 mm in diameter, 37 nodules were 3 mm to 5 mm, and 57 nodules were >5 mm. A total of 137 metastatic nodules were detected by the 1.5 T MRI with a double dose of Gd-DTPA in the final interpretation. Of these nodules, 43 nodules were ≤ 3 mm in diameter. The 3.0 T MRI with a double dose of Gd-DTPA was significantly more effective at detecting nodules smaller than 3 mm (p = 0.014) than the 1.5 T MRI. However, we found no significant difference in the detection of nodules larger than 3 mm (p > 0.05) (Table 1). In 6 of the 31 cases, six nodules, which were only detected by the 3.0 T MRI with a double dose of Gd[1]DTPA, and missed by the 1.5 T MRI with a double dose of Gd-DTPA, were unanimously agreed upon by all participants in the final interpretation. In three of the six nodules, a growing nodule was detected during a follow-up MR scan which confirmed the presence of metastases (Fig. 1). Three of the 6 nodules missed were unable to be detected using the 1.5 T MRI with a double dose of Gd-DTPA due to artifact (Fig. 2). Finally the 3.0 T MRI with a double dose of Gd-DTPA was Stissue1 – Stissue2 Stissue2 Evaluation between 3.0 T vs 1.5 T MRI in Detection of Brain Metastasis using Double Dose Gd-DTPA – 103 – Table 1. Total Number of Brain Metastases Classified by Size and Detected at the Final Interpretation Size 3.0 T MRI with 1.5 T MRI with p value Gd-DTPA Gd-DPTA ≤ 3 mm 49 43 0.014 3 mm<-≤5 mm 37 37 > 0.05 5 mm< 57 57 > 0.05 Total 143 137 0.014 Note : Data are the number of nodules Table 2. The Values of Currently Used Statistical Measures for the 3.0 T and 1.5 T MRIs with Gd-DTPA According to Nodule Size Metastatic nodules Total ≤ 3 mm Present Absent Present Absent 3.0 T MRI with Positive 143 00* 49 0* Gd-DTPA Negatiive 000 00* 00 0* 1.5 T MRI with Positive 137 18* 43 18* Gd-DTPA Negative 006 00* 06 0* * Pseudolesions that were detected as nodules by the 1.5 T MRI were detected as vascular structures by the 3.0 T MRI. useful for confirming the presence of these nodules. Nodules observed by the 3.0 T MRI with a double dose of Gd-DTPA were brighter or better delineation than by the 1.5 T MRI with a double dose of Gd-DTPA. Out of 137 nodules, 65 were brighter (Fig. 3) and out of 137 nodules, 63 were better delineated (Fig. 4). Forty[1]five of 137 nodules were satisfactory in both conditions. By consensus, the final review stated, nodules detected by the 3.0 T and 1.5 T MRI were considered positive for metastases (Table 2). Additionally, there were 18 pseudolesions which were detected as nodules by the 1.5 T MRI, but as vascular structures by the 3.0 T MRI (Fig. 5). The sensitivity, positive predictive values, and diagnostic accuracy of the 1.5 T MRI with a double dose of Gd-DTPA were 95.8%, 88.3%, and 85.1% respectively. The sensitivity, positive predictive value, and diagnostic accuracy of the 3.0 T MRI with a double dose of Gd-DTPA were 100%, 100%, and Woo-Suk Chung et al – 104 – a b Fig. 2.
A 73-year-old male with multiple brain metastases. Metastatic nodule (a) by a 3.0 T MR image with a double dose of Gd-DTPA show a metastatic nodule (arrow). But (b) the 1.5 T MR image with a double dose of Gd-DTPA cannot show the metastatic nodule by artifact. abc Fig. 1. A 65-year-old male with single brain metastasis. (a) The 3.0 T MR image with a double dose of Gd-DTPA shows a small metastatic nodule (arrow). (b) The 1.5 T MR image with a double dose of Gd-DTPA cannot show the nodule. In (c), a growing nodule was detected during the follow-up MRI with a double dose of Gd-DTPA which confirmed the presence of metastasis (arrow). 100% respectively. For metastatic nodules smaller than 3 mm, the sensitivity, positive predictive value, and diagnostic accuracy of the 1.5 T MRI with a double dose of Gd-DTPA were 87.8%, 70.5%, and 64.2% respectively. The sensitivity, positive predictive value, and diagnostic accuracy of 3.0 T MRI with a double dose of Gd-DTPA were 100%, 100%, and 100% respectively (Table 3). For quantitative image assessment, the SI ratio in the post contrast sequences (Table 4) was significantly higher in the 3.0 T MRI with a double dose of Gd[1]DTPA than in the 1.5 T MRI with a double dose of Gd[1]DTPA (p = 0.025). Discussion Detection of metastatic nodules is dependent on both their size and contrast ratio (2). As methods to increase the contrast, a higher dose of Gd-DTPA, a higher field strength, and delayed study can all be used to aid detection (2-5, 8-17). Nodules larger than 10 mm are easily detected because vasogenic edema is customarily associated with larger metastases (2). So, when a higher dose of Gd-DTPA is used, the detection rate of larger nodules is not influenced. However, a higher dose is helpful for detecting small nodules because it increases nodule enhancement, yet this method has the disadvantages of increasing the false positive rate and promoting side effects (2-5, 8-14).
A delayed study can also increase the contrast (2). It has been recommended that image acquisition be delayed from 5 to 35 minutes after the administration of contrast material at a dose of 0.1 mmol/kg to ensure optimal detection (2, 15). Three of the 6 nodules missed were unable to be detected using the 1.5 T MRI with a double dose of Gd[1]DTPA due to partial volume artifact. Eighteen lesions were detected in the 1.5 T MRI with a double dose of Gd-DTPA, which proved to be vascular structures in 3.0 T MRI with a double dose of Gd- DTPA. The true nature of these lesions was revealed because of the greater morphologic detail visualized by the high field Evaluation between 3.0 T vs 1.5 T MRI in Detection of Brain Metastasis using Double Dose Gd-DTPA – 105 – a b Fig. 3. A 65-year-old male with multiple brain metastases. (a) The 3.0 T MR image with a double dose of Gd-DTPA shows a metastatic nodule with spherical enhancement (arrow). (b) The 1.5 T MR image with a double dose of Gd-DTPA shows a metastatic nodule with spherical enhance[1]ment (arrow). Nodules observed by the 3.0 T MRI with a double dose of Gd-DTPA were brighter than when observed by the 1.5 T MRI with a double dose of Gd[1]DTPA. Table 3. Estimation of the Values of Currently Used Statistical Measures for the 3.0 T and 1.5 T MRI with Gd-DTPA According to Nodule Size Metastatic nodules Total ≤ 3 mm 3.0 T 1.5T 3.0 T 1.5T Sensitivity 100 95.8 100 87.8 Specificity – 0 – 0 Positive predictive value 100 88.3 100 70.5 Negative predictive value – 0 – 0 Diagnostic accuracy 100 85.1 100 64.2 Table 4. Results of a quantitative assessment of nodules that were detected by the 3.0 T and 1.5 T MRIs with Gd-DTPA (n=137) 3.0 T 1.5 T P value Nodules-to-adjacent tissue: SI ratio 94.59 63.86 0.025 strength MR image, thus allowing differentiation between true enhancing lesions and sulcal vessels. In our study, each nodules was classified according to its largest diameter as being ≤ 3 mm, 3 mm to 5 mm, and >5 mm because the slice thickness of the 3.0 T MRI was 2.0 mm and 5 mm for the 1.5 T MRI. Therefore this study classified small metastatic nodules as being smaller than 3 mm. Although our study supports the use of a higher field strength MRI with a double dose of Gd-DTPA for increased metastatic nodule detection and for improved nodule enhancement and delineation, the results should be interpretated with caution.
The reason for this is that for patients with two or more brain metastases, additional metastases found with the 3.0 T MRI seem to be of limited clinical importance. The presence of two or more small nodules generally will not change the way the patient is managed. Therefore, it is of utmost importance to identify the difference between none, one, and more than one metastatic nodule.
Patients with a single metastatic nodule located in a respectable region can be treated surgically, and the tumor staged as M1, not M0. However, patients with two or more metastatic nodules are usually Woo-Suk Chung et al – 106 – a b Fig. 5. A 67-year-old female with multiple brain metastases. (a) The 3.0 T MRI with a double dose of Gd-DTPA shows vascular structure (arrow). (b) The 1.5 T MRI with a double dose of Gd[1]DTPA shows a nodular lesion (arrow). A pseudolesion that was detected as a nodule by the 1.5 T MRI with a double dose of Gd[1]DTPA but as a vascular structure by the 3.0 T MRI with a double dose of Gd-DTPA is showed in this figure. a b Fig. 4. A 64-year-old male with multiple brain metastases. (a) The 3.0 T MRI with a double dose of Gd-DTPA shows a metastatic nodule with ring enhancement (arrow). (b) The 1.5 T MRI with Gd-DTPA show a metastatic nodule with ring enhancement (arrow). Nodules observed by the 3.0 T MRI with a double dose of Gd-DTPA were better delineated than when observed by the 1.5 T MRI with a double dose of Gd[1]DTPA. treated with radiation therapy and/or systemic chemotherapy (3, 18-22). On the other hand, the use of a higher strength field MRI was found to be helpful in confirming the appearance of an equivocal metastatic nodule. The 3.0 T MRI with a double dose of Gd-DTPA was also useful for the detection of additional metastases in patients with a known lesion detected by the 1.5 T MRI with a double dose of Gd[1]DTPA. Because this also has influence on the sensitivity, positive predictive value, and diagnostic accuracy the 3.0 T MRI with a double dose of Gd[1]DTPA was found to have better results than the 1.5 T MRI with a double dose of Gd-DTPA in our study. Therefore we recommend the use of the 3.0 T MRI with a double dose of Gd-DTPA in only three circumstances: when the findings by the 1.5 T MRI with Gd-DTPA are equivocal, when one potentially surgically respectable nodule is identified, or for detecting early and/or small metastases
There were two limitations in this study. The first limitation was a difference in protocol sequence. The T1 weighted spin-echo protocol was used for the 1.5 T MRI and SPGR technique protocol for the 3.0 T MRI. Detectability of metastatic nodules is more effective with 3.0 T MRI with T1 SE than with a 1.5 T MRI with SE (2). SPGR with thin slice thickness is superior to the T1 spin echo sequence with thick slice thickness due to a partial volume effect. Additionally, SE is not optimum for a 3.0 T due to longer T1- and shorter T2- relaxation times of water protons, which decrease the contrast ratio in the 3.0 T images.17 The purpose of this study was to detect early small brain metastases, so using the SPGR sequence with a 3.0 T MRI, which is usually used at present, has advantages for detecting early small brain metastases. The second limitation was that the scan interval between the 1.5 T and 3.0 T MRI was less than 20 minutes. However, a delayed study increases contrast as mentioned above. The current study found that the SI ratio was significantly higher in the 3.0T images than in the 1.5 T images. Considering the delayed study by the 1.5 T MRI, this limitation emphasizes the better detection rate of the 3.0 T MRI with a double dose of Gd-DTPA. And due to these confounding variables, blinded reviews were performed by three readers. The detectability of metastatic nodules smaller than 3mm was better using a 3.0 T MRI with SPGR than a 1.5 T MRI with T1 SE. Therefore we recommend a 3.0 T MRI with SPGR and a double dose of Gd-DTPA for detecting early and/or small metastatic nodules, furthermore influencing treatment. References 1.Lassman AB, DeAngelis LM. Brain metastases. Neurol Clin. 2003; 21:1-23, vii, Review 2.Ba-Ssalamah A, Nobauer-Huhmann IM, Pinker K, et al. Effect of contrast dose and field strength in the magnetic resonance detection of brain metastases. Invest Radiol 2003; 38: 415-422 3.Sze G, Johnson C, Kawamura Y, et al. Comparison of single[1]and triple-dose contrast material in the MR screening of brain metastases. AJNR Am J Neuroradiol 1998; 19:821-828 4.Runge VM, Kirsch JE, Burke VJ, et al. High-dose Gadoteridol in MR imaging of intracranial neoplasm. J Magn Reson Imaging 1992; 2:9-18 5.Yuh WT, Engelken JD, Muhonen MG, Mayr NA, Fisher DJ, Ehrhardt JC. Experience with high-dose gadolinium MR imaging in the evaluation of brain metastases. AJNR Am J Neuroradiol 1992; 13:335-345 6.Brekenfeld C, Foert E, Hundt W, Kenn W, Lodeann KP, Gehl HB. Enhancement of cerebral diseases: How much contrast agent is enough? Comparison of 0.1, 0.2, and 0.3 mmol/kg Gadoteridol at 0.2 T with 0.1 mmol/kg Gadoteridol at 1.5 T. Invest Radiol 2001; 36:266-275 7.Yuh WT, Tali ET, Nguyen HD, Simonson TM, Mayr NA, Fisher DJ. The effect of contrast dose, imaging time, and lesion size in the MR detection of intracerebral metastasis. AJNR Am J Neuroradiol 1998; 16:373-380 8.Van Dijk P, Sijens PE, Schmitz PI, Oudkerk M. Gd-enhanced MR imaging of brain metastases: contrast as a function of dose and lesion size. Magn Reson Imaging 1997; 15:535-541 9.Yuh WT, Fisher DJ, Engelken JD, et al. MR evaluation of CNS tumors: dose comparison study with gadopentetate dimeglumine and gadoteridol. Radiology 1991; 180:485-491 10.Healy ME, Hasselink JR, Press GA, Middleton MS. Increased detection of intracranial metastases with intravenous Gd[1]DTPA. Radiology 1987; 165:619-624 11.Akeson P, Vikhoff B, Stahlberg F, Holtas S. Brain lesion contrast in MR imaging: dependence of field strength and concentration of gadodiamide injection in patients and phantoms. Acta Radiol 1977; 38:14-18 12.Runge VM. A review of contrast media research in 1999 – 2000. Invest Radiol 2001; 36:123-130 13.Yuh WT, Parker JR Carvlin MJ. Indication related dosing for magnetic resonance contrast media. Eur Radiol 1977; 7(suppl 5):S269-S275 14.Rinck PA, Muller RN. Field strength and dose dependence of contrast enhancement by Gadolinium-based MR contrast agents. Eur Radiol 1999; 9:998-1004 15.Schorner W, Laniado M, Niendorf HP, Schubert C, Felix R. Time-dependent changes in image contrast in brain tumors after gadolinium-DTPA. AJNR Am J Neuroradiol 1986; Evaluation between 3.0 T vs 1.5 T MRI in Detection of Brain Metastasis using Double Dose Gd-DTPA – 107 – 7:1013-1020 16.Chang KH, Ra DG, Han MH, Cha SH, Kim HD, Han MC. Contrast enhancement of brain tumors at different MR field strengths: comparison of 0.5 T and 2.0 T. AJNR Am J Neuroradiol 1994; 15:1413-1419 17.Nobauer-Huhmann IM, Ba-Ssalamah A, Mlynarik V, et al. Magnetic resonance imaging contrast enhancement of brain tumors at 3 tesla versus 1.5 tesla. Invest Radiol 2002; 37:114- 119 18.Galicich JH, Sundaresan N, Thaler HT. Surgical treatment of single brain metastasis: evaluation of results by computerized tomography scanning. J Neurosurg 1980; 53:63-67 19.Runge VM, Carollo BR, Wolf CR, Nelson KL, Gelblum DY. Gd-DTPA: A review of clinical indications in central nervous system magnetic resonance imaging. Radiographics 1989; 9:929-958 20.Earnest F 4th, Ryu JH, Miller GM, et al. Suspected non-small cell lung cancer: Incidence of occult brain and skeletal metastases and effectiveness of imaging for detection-Pilot study. Radiology 1999; 211:137-145 21.Koutras AK, Marangos M, Kourelis T, et al. Surgical management of cerebral metastases from non-small cell lung cancer. Tumori 2003; 89:292-297 22.Soffietti R, Ruda R, Mutani R. Management of brain metastases. J Neurol 2002; 249:1357-1369. Review Woo-Suk Chung et al – 108 – 통신저자 : 정태섭, 서울특별시 강남구 도곡동 146-92 연세대학교 영동세브란스병원 영상의학과 Tel. 82-2019-3514 Fax. 82-3462-5472 E-mail: tschung@yumc.yonsei.ac.kr 뇌전이종양의발견에있어서Doble dose Gd-DTPA를이용한 3 T MRI와1.5 T MRI간의비교연구 1 연세대학교 영동세브란스병원 영상의학과 2 연세대학교 영동세브란스병원 호흡기내과 정우석1 ·정태섭1 ·김형중2 ·안철민2 ·이재훈1 ·허 진1 ·조응혁1 목적: 작은 뇌전이 종양의 조기 발견은 중요하다. 이 연구의 목적은 1.5 T MRI와 3.0 T MRI 간의 크기에 따른 뇌 전이 종양의 발견율을 비교하는 것이다. 대상 및 방법: 폐암으로 진단 받은 162명의 환자를 대상으로 TNM 병기를 위해 뇌 MRI를 시행하였다. Gd-DTPA 를 2배 용량으로 투여 후, 3.0 T MRI에서 훼손경사회복획득으로 촬영하였으며 그 후 1.5 T MRI에서 T1 스핀 에 코로 촬영하였다. 3명의 방사선과 전문의가 합의하여 MRI를 판독하였으며 정성 평가를 시행하였다. 3.0 T와 1.5 T MRI에서 크기에 따라 민감도, 양성 예측률, 정확도를 평가하였다. 신호 강도를 사용하여 전이 종양과 인접 조직간 의 신호강도 비를 계산하였다. 결과: 162명의 환자 중 31명에서 1.5 T 또는 3.0 T MR에 뇌전이 종양이 발견되었다. 3.0 T MRI에서 143개의 종 양이 발견되었으나 1.5 T MRI에서 137개의 종양이 발견되었다. 6개의 종양이 3.0 T MRI에서만 발견되었으며 크 기는 모두 3 mm 미만이었다. 3.0 T MRI의 민감도, 양성 예측률, 정확도는 각각 100%, 100%, 100%이며, 1.5 T MRI에서는 각각 95.8%, 88.3%, 85.1% 이다. SI ratio는 1.5 T MRI보다 3.0 T MRI에서 유의하게 높았다 (p=0.025). 결론: Double dose Gd-DTPA를 이용한 3.0 T MRI는 3 mm미만의 뇌전이 종양을 발견하는데 있어서 1.5 T
Evaluation between 3.0 T vs 1.5 T MRI in Detection of
Brain Metastasis using Double Dose Gd-DTPA
Woo-Suk Chung, M.D.1
, Tae-Sub Chung, M.D.1
, Hyung Jung Kim, M.D.2
, Chul Min Ahn,
M.D.2
, Jae Hoon Lee, M.D.1
, Jin Hur, M.D.1
, Arthur Eung-Hyuck Cho, M.D.1
Purpose : Early detection of small brain metastases is important. The purpose of this
study was to compare the detectability of brain metastases according to the size
between 1.5 T and 3.0 T MRI.
Materials and Methods : We reviewed 162 patients with primary lung cancer who
were examined for TNM staging. After administration of double dose of Gd-DTPA,
MR imaging was performed with SPGR by 3.0 T MRI and then with T1 SE sequence
by 1.5 T MRI. In each patient, three readers performed qualitative assessment.
Sensitivity, positive predictive value, and diagnostic accuracy were calculated in 3.0
T and 1.5 T MRI according to size. Using the signal intensity (SI) measurements
between the metastatic nodules and adjacent tissue, nodule-to-adjacent tissue SI ratio
was calculated.
Results : Thirty-one of 162 patients had apparent metastatic nodules in the brain at
either 1.5 T or 3.0 T MR imaging. 143 nodules were detected in 3.0 T MRI, whereas
137 nodules were detected at 1.5 T MRI. Six nodules, only detected in 3.0 T MRI,
were smaller than 3.0 mm in dimension. Sensitivity, positive predictive value, and
diagnostic accuracy in 3.0 T MRI were 100 %, 100 %, and 100 % respectively, and
in 1.5 T MRI were 95.8 %, 88.3 %, and 85.1 % respectively. SI ratio was significantly
higher in the 3.0 T MRI than 1.5 T MRI (p=0.025).
Conclusion : True positive rate of 3.0 T MRI with Gd-DTPA was superior to 1.5 T
MRI with Gd-DTPA in detection of metastatic nodules smaller than 3.0 mm.
Index words :Magnetic resonance (MR), high-field-strength imaging
Brain neoplasm, metastases
Contrast medium
Introduction
Brain metastases present a poor prognosis suggesting
a shortened survival time. Early diagnosis of brain
involvement and determination of the number of
metastases are important not only for quality of life but
also for cost effectiveness (1-3). The decision regarding
a conservative versus a surgical approach depends on
the number of brain metastases detected by radiologic
means (1-3).
A contrast-enhanced MRI has become the method of
choice for visualization of brain metastases (1). Highdose gadolinium-enhanced MR examinations may have
advantages over 0.1 mmol/kg examinations in detecting
early and/or small metastases (3-7). A delayed study
can also increase the contrast (2). Comparing to the 1.5
T MRI with an axial T1-weighted spin echo sequence
(SE), the higher field strength MR systems combined
with a sequence of SPGR (spoiled gradient recalled
acquisition in the steady state) and administration of a
high dose of Gd-DTPA (gadopentetate dimeglumine)
may also have advantages in detecting small
metastases, although this has not yet been verified by
clinical data. The purpose of this study is to compare
the detectability of brain metastases classified
according to the size of nodules, between thin slice
SPGR of 3.0 T and conventional thick slice SE of 1.5 T
MRI with the administration of a double dose of GdDTPA.
Materials and Methods
Patients
From December 2002 to February 2004, a total of 162
consecutive patients with primary lung cancer
participated in our study. The institutional review
board approved our study, and informed consent was
obtained from all patients regarding the potential risks
of both the double dose of contrast medium and two
assessments by MRI scanning on a 3.0 T and 1.5 T
machine. After the study, 31 patients of this population
were diagnosed as having brain metastases. Mean
patient age was 61.2 (range, 43 to 80 years).
Protocol
On the 3.0 T MR scanner (GE Signa VH/i; GE
medical system, Milwaukee, USA), the images were
acquired using a standard head coil and an actively
shielded gradient system with a maximum gradient
strength of 43 mT/m. On the 1.5 T MR scanner
(Magnetom Vision; Siemens Medical Systems,
Erlangen, Germany), a standard head coil and a
maximum gradient strength of 25 mT/m were used.
All patients were examined after administrating a
contrast agent with a double dose of Gd-DTPA (0.2
mmol/kg). First, examinations were performed on a 3.0
T MR scanner and then were subsequently performed
on a 1.5 T MR scanner without additional contrast
injection. The scan interval between the 3.0 T and 1.5
T MR examination was less than 20 minutes. The
contrast agent used was Gd-DTPA (Magnevist;
Schering AG, Berlin, Germany). In all patients, the
double dose of Gd-DTPA at 0.2 mmol/kg was
administered intravenously as a bolus and scanning
commenced immediately.
MR imaging included the following sequences on
both scanners: At 3.0 T MRI, an axial 3D SPGR, which
is usually used at present, was used (TR/TE/TI =
5.7/1.44/400 milliseconds; flip angle 20°; 2 mm slice
thickness; FOV of 220 mm; matrix size of 512×512
ZIP; spatial resolution of 0.43×0.43×2 mm; two
acquisition) with a scan time of 3 minutes 30 seconds.
At 1.5 T MRI, an axial T1-weighted spin echo sequence
was used (TR/RE = 600/14 milliseconds; flip angle 90°;
5 mm slice thickness; FOV of 210 mm; matrix size of
174×256; spatial resolution of 1.2×0.82×5 mm; two
acquisition) with a scan time of 5 minutes 30 seconds.
Three radiologists performed randomized,
independent blinded review. The three readers were
merely informed that all of the patients had lung
malignancies. The postcontrast MR examinations of the
brain on all of the patients were evaluated. The readers
did not have access to other image sets within each
study.
The presence, size, and number of metastatic nodules
were assessed. The postcontrast images were divided
into the following two groups: 3.0 T MRI with a double
dose of Gd-DTPA and 1.5 T MRI with a double dose
of Gd-DTPA. The readers were asked to document the
number of nodules. Each nodule in each study was
numbered and classified according to its largest
diameter measurement: ≤3 mm, 3 mm to 5 mm, or
5 mm.
Woo-Suk Chung et al
- 102 –
Subsequently, if there was any debate about nodule
classification, a final interpretation using imaging
studies was done until consensus among the readers
was accomplished. If disagreements persisted, 3-6
months of follow-up MR scans were obtained for
further validation. For the patients who had follow-up
MR scans, these scans were considered positive for
metastases if there was a response to the treatment or
if there was a growth of nodules identified during the
follow-up period.
In addition, the sensitivity, specificity, positive
predictive value, negative predictive value, and
diagnostic accuracy of the 3.0 T and 1.5 T MRI were
calculated using the results from the final interpretation
according to size.
A quantitative image assessment was performed next.
The same lesions on scans of the two different MRI
systems were determined by comparing peripheral
structures. Signal intensities of adjacent tissue and
nodule were assessed by region of interest (ROI)
measurements placed identically on both series of
images using the same sized circular ROI from the
software available on both scanners. Nodule-toadjacent tissue contrast (SI ratio) is defined by
SI ratio = ×100
Stissue1 taken as the signal intensity of an ROI assessed
over a nodule and Stissue2 taken as the signal intensity
assessed over the contralateral white matter. The signal
intensity of a nodule was measured within an
enhanced area. In inhomogeneously enhanced nodules,
the area of maximum uptake was chosen for
measurement.
Statistical Analysis
The paired Wilcoxon’s signed ranks test was used to
compare qualitative scores, and the matched-pair-t test
was used to compare nodule SI ratios between the 3.0
T and 1.5 T MRI with a double dose of Gd-DTPA. For
all tests, significance was set at p < 0.05, and SPSS software (SPSS Inc. Chicago, US) was used for statistical analyses. Results In the final review made by consensus of three radiologists (Table 1), a total of 143 metastatic nodules were detected by the 3.0 T MRI with a double dose of Gd-DTPA. Of these nodules, 49 nodules were ≤ 3 mm in diameter, 37 nodules were 3 mm to 5 mm, and 57 nodules were >5 mm. A total of 137 metastatic
nodules were detected by the 1.5 T MRI with a double
dose of Gd-DTPA in the final interpretation. Of these
nodules, 43 nodules were ≤ 3 mm in diameter. The
3.0 T MRI with a double dose of Gd-DTPA was
significantly more effective at detecting nodules smaller
than 3 mm (p = 0.014) than the 1.5 T MRI. However,
we found no significant difference in the detection of
nodules larger than 3 mm (p > 0.05) (Table 1).
In 6 of the 31 cases, six nodules, which were only
detected by the 3.0 T MRI with a double dose of GdDTPA, and missed by the 1.5 T MRI with a double
dose of Gd-DTPA, were unanimously agreed upon by
all participants in the final interpretation. In three of
the six nodules, a growing nodule was detected during
a follow-up MR scan which confirmed the presence of
metastases (Fig. 1). Three of the 6 nodules missed were
unable to be detected using the 1.5 T MRI with a
double dose of Gd-DTPA due to artifact (Fig. 2). Finally
the 3.0 T MRI with a double dose of Gd-DTPA was
Stissue1 – Stissue2
Stissue2
Evaluation between 3.0 T vs 1.5 T MRI in Detection of Brain Metastasis using Double Dose Gd-DTPA- 103 –
Table 1. Total Number of Brain Metastases Classified by Size
and Detected at the Final Interpretation
Size 3.0 T MRI with 1.5 T MRI with p value Gd-DTPA Gd-DPTA
≤ 3 mm 49 43 0.014
3 mm<-≤5 mm 37 37 > 0.05
5 mm< 57 57 > 0.05
Total 143 137 0.014
Note : Data are the number of nodules
Table 2. The Values of Currently Used Statistical Measures for
the 3.0 T and 1.5 T MRIs with Gd-DTPA According to Nodule
Size
Metastatic nodules
Total ≤ 3 mm
Present Absent Present Absent
3.0 T MRI with Positive 143 00* 49 0*
Gd-DTPA Negatiive 000 00* 00 0*
1.5 T MRI with Positive 137 18* 43 18*
Gd-DTPA Negative 006 00* 06 0*- Pseudolesions that were detected as nodules by the 1.5 T MRI
were detected as vascular structures by the 3.0 T MRI.
useful for confirming the presence of these nodules.
Nodules observed by the 3.0 T MRI with a double
dose of Gd-DTPA were brighter or better delineation
than by the 1.5 T MRI with a double dose of Gd-DTPA.
Out of 137 nodules, 65 were brighter (Fig. 3) and out of
137 nodules, 63 were better delineated (Fig. 4). Fortyfive of 137 nodules were satisfactory in both conditions.
By consensus, the final review stated, nodules
detected by the 3.0 T and 1.5 T MRI were considered
positive for metastases (Table 2). Additionally, there
were 18 pseudolesions which were detected as nodules
by the 1.5 T MRI, but as vascular structures by the 3.0
T MRI (Fig. 5). The sensitivity, positive predictive
values, and diagnostic accuracy of the 1.5 T MRI with
a double dose of Gd-DTPA were 95.8%, 88.3%, and
85.1% respectively. The sensitivity, positive predictive
value, and diagnostic accuracy of the 3.0 T MRI with
a double dose of Gd-DTPA were 100%, 100%, and
Woo-Suk Chung et al- 104 –
a b
Fig. 2. A 73-year-old male with multiple brain metastases. Metastatic nodule (a) by a 3.0 T MR image with a double
dose of Gd-DTPA show a metastatic nodule (arrow). But (b) the 1.5 T MR image with a double dose of Gd-DTPA
cannot show the metastatic nodule by artifact.
abc
Fig. 1. A 65-year-old male with single brain metastasis. (a) The 3.0 T MR image with a double dose of Gd-DTPA
shows a small metastatic nodule (arrow). (b) The 1.5 T MR image with a double dose of Gd-DTPA cannot show
the nodule. In (c), a growing nodule was detected during the follow-up MRI with a double dose of Gd-DTPA which
confirmed the presence of metastasis (arrow).
100% respectively. For metastatic nodules smaller than
3 mm, the sensitivity, positive predictive value, and
diagnostic accuracy of the 1.5 T MRI with a double
dose of Gd-DTPA were 87.8%, 70.5%, and 64.2%
respectively. The sensitivity, positive predictive value,
and diagnostic accuracy of 3.0 T MRI with a double
dose of Gd-DTPA were 100%, 100%, and 100%
respectively (Table 3).
For quantitative image assessment, the SI ratio in the
post contrast sequences (Table 4) was significantly
higher in the 3.0 T MRI with a double dose of GdDTPA than in the 1.5 T MRI with a double dose of GdDTPA (p = 0.025).
Discussion
Detection of metastatic nodules is dependent on both
their size and contrast ratio (2). As methods to increase
the contrast, a higher dose of Gd-DTPA, a higher field
strength, and delayed study can all be used to aid
detection (2-5, 8-17). Nodules larger than 10 mm are
easily detected because vasogenic edema is customarily
associated with larger metastases (2). So, when a higher
dose of Gd-DTPA is used, the detection rate of larger
nodules is not influenced. However, a higher dose is
helpful for detecting small nodules because it increases
nodule enhancement, yet this method has the
disadvantages of increasing the false positive rate and
promoting side effects (2-5, 8-14). A delayed study
can also increase the contrast (2). It has been
recommended that image acquisition be delayed from
5 to 35 minutes after the administration of contrast
material at a dose of 0.1 mmol/kg to ensure optimal
detection (2, 15).
Three of the 6 nodules missed were unable to be
detected using the 1.5 T MRI with a double dose of GdDTPA due to partial volume artifact. Eighteen lesions
were detected in the 1.5 T MRI with a double dose of
Gd-DTPA, which proved to be vascular structures in
3.0 T MRI with a double dose of Gd- DTPA. The true
nature of these lesions was revealed because of the
greater morphologic detail visualized by the high field
Evaluation between 3.0 T vs 1.5 T MRI in Detection of Brain Metastasis using Double Dose Gd-DTPA- 105 –
a b
Fig. 3. A 65-year-old male with
multiple brain metastases. (a) The
3.0 T MR image with a double
dose of Gd-DTPA shows a
metastatic nodule with spherical
enhancement (arrow). (b) The 1.5
T MR image with a double dose
of Gd-DTPA shows a metastatic
nodule with spherical enhancement (arrow). Nodules observed
by the 3.0 T MRI with a double
dose of Gd-DTPA were brighter
than when observed by the 1.5 T
MRI with a double dose of GdDTPA.
Table 3. Estimation of the Values of Currently Used Statistical
Measures for the 3.0 T and 1.5 T MRI with Gd-DTPA According
to Nodule Size
Metastatic nodules
Total ≤ 3 mm
3.0 T 1.5T 3.0 T 1.5T
Sensitivity 100 95.8 100 87.8
Specificity – 0 – 0
Positive predictive value 100 88.3 100 70.5
Negative predictive value – 0 – 0
Diagnostic accuracy 100 85.1 100 64.2
Table 4. Results of a quantitative assessment of nodules that
were detected by the 3.0 T and 1.5 T MRIs with Gd-DTPA
(n=137)
3.0 T 1.5 T P value
Nodules-to-adjacent tissue: SI ratio 94.59 63.86 0.025
strength MR image, thus allowing differentiation
between true enhancing lesions and sulcal vessels.
In our study, each nodules was classified according
to its largest diameter as being ≤ 3 mm, 3 mm to 5
mm, and >5 mm because the slice thickness of the 3.0
T MRI was 2.0 mm and 5 mm for the 1.5 T MRI.
Therefore this study classified small metastatic nodules
as being smaller than 3 mm.
Although our study supports the use of a higher field
strength MRI with a double dose of Gd-DTPA for
increased metastatic nodule detection and for improved
nodule enhancement and delineation, the results
should be interpretated with caution. The reason for
this is that for patients with two or more brain
metastases, additional metastases found with the 3.0 T
MRI seem to be of limited clinical importance. The
presence of two or more small nodules generally will
not change the way the patient is managed. Therefore,
it is of utmost importance to identify the difference
between none, one, and more than one metastatic
nodule. Patients with a single metastatic nodule located
in a respectable region can be treated surgically, and
the tumor staged as M1, not M0. However, patients
with two or more metastatic nodules are usually
Woo-Suk Chung et al- 106 –
a b
Fig. 5. A 67-year-old female with
multiple brain metastases. (a) The
3.0 T MRI with a double dose of
Gd-DTPA shows vascular
structure (arrow). (b) The 1.5 T
MRI with a double dose of GdDTPA shows a nodular lesion
(arrow). A pseudolesion that was
detected as a nodule by the 1.5 T
MRI with a double dose of GdDTPA but as a vascular structure
by the 3.0 T MRI with a double
dose of Gd-DTPA is showed in
this figure.
a b
Fig. 4. A 64-year-old male with
multiple brain metastases. (a) The
3.0 T MRI with a double dose of
Gd-DTPA shows a metastatic
nodule with ring enhancement
(arrow). (b) The 1.5 T MRI with
Gd-DTPA show a metastatic
nodule with ring enhancement
(arrow). Nodules observed by the
3.0 T MRI with a double dose of
Gd-DTPA were better delineated
than when observed by the 1.5 T
MRI with a double dose of GdDTPA.
treated with radiation therapy and/or systemic
chemotherapy (3, 18-22). On the other hand, the use
of a higher strength field MRI was found to be helpful
in confirming the appearance of an equivocal
metastatic nodule. The 3.0 T MRI with a double dose
of Gd-DTPA was also useful for the detection of
additional metastases in patients with a known lesion
detected by the 1.5 T MRI with a double dose of GdDTPA. Because this also has influence on the
sensitivity, positive predictive value, and diagnostic
accuracy the 3.0 T MRI with a double dose of GdDTPA was found to have better results than the 1.5 T
MRI with a double dose of Gd-DTPA in our study.
Therefore we recommend the use of the 3.0 T MRI
with a double dose of Gd-DTPA in only three
circumstances: when the findings by the 1.5 T MRI
with Gd-DTPA are equivocal, when one potentially
surgically respectable nodule is identified, or for
detecting early and/or small metastases
There were two limitations in this study. The first
limitation was a difference in protocol sequence. The
T1 weighted spin-echo protocol was used for the 1.5 T
MRI and SPGR technique protocol for the 3.0 T MRI.
Detectability of metastatic nodules is more effective
with 3.0 T MRI with T1 SE than with a 1.5 T MRI with
SE (2). SPGR with thin slice thickness is superior to the
T1 spin echo sequence with thick slice thickness due
to a partial volume effect. Additionally, SE is not
optimum for a 3.0 T due to longer T1- and shorter T2-
relaxation times of water protons, which decrease the
contrast ratio in the 3.0 T images.17 The purpose of
this study was to detect early small brain metastases,
so using the SPGR sequence with a 3.0 T MRI, which
is usually used at present, has advantages for detecting
early small brain metastases. The second limitation was
that the scan interval between the 1.5 T and 3.0 T MRI
was less than 20 minutes. However, a delayed study
increases contrast as mentioned above. The current
study found that the SI ratio was significantly higher in
the 3.0T images than in the 1.5 T images. Considering
the delayed study by the 1.5 T MRI, this limitation
emphasizes the better detection rate of the 3.0 T MRI
with a double dose of Gd-DTPA. And due to these
confounding variables, blinded reviews were
performed by three readers.
The detectability of metastatic nodules smaller than
3mm was better using a 3.0 T MRI with SPGR than a
1.5 T MRI with T1 SE. Therefore we recommend a 3.0
T MRI with SPGR and a double dose of Gd-DTPA for
detecting early and/or small metastatic nodules,
furthermore influencing treatment.
References
1.Lassman AB, DeAngelis LM. Brain metastases. Neurol Clin.
2003; 21:1-23, vii, Review
2.Ba-Ssalamah A, Nobauer-Huhmann IM, Pinker K, et al. Effect
of contrast dose and field strength in the magnetic resonance
detection of brain metastases. Invest Radiol 2003; 38: 415-422
3.Sze G, Johnson C, Kawamura Y, et al. Comparison of singleand triple-dose contrast material in the MR screening of brain
metastases. AJNR Am J Neuroradiol 1998; 19:821-828
4.Runge VM, Kirsch JE, Burke VJ, et al. High-dose Gadoteridol
in MR imaging of intracranial neoplasm. J Magn Reson
Imaging 1992; 2:9-18
5.Yuh WT, Engelken JD, Muhonen MG, Mayr NA, Fisher DJ,
Ehrhardt JC. Experience with high-dose gadolinium MR
imaging in the evaluation of brain metastases. AJNR Am J
Neuroradiol 1992; 13:335-345
6.Brekenfeld C, Foert E, Hundt W, Kenn W, Lodeann KP, Gehl
HB. Enhancement of cerebral diseases: How much contrast
agent is enough? Comparison of 0.1, 0.2, and 0.3 mmol/kg
Gadoteridol at 0.2 T with 0.1 mmol/kg Gadoteridol at 1.5 T.
Invest Radiol 2001; 36:266-275
7.Yuh WT, Tali ET, Nguyen HD, Simonson TM, Mayr NA,
Fisher DJ. The effect of contrast dose, imaging time, and
lesion size in the MR detection of intracerebral metastasis.
AJNR Am J Neuroradiol 1998; 16:373-380
8.Van Dijk P, Sijens PE, Schmitz PI, Oudkerk M. Gd-enhanced
MR imaging of brain metastases: contrast as a function of
dose and lesion size. Magn Reson Imaging 1997; 15:535-541
9.Yuh WT, Fisher DJ, Engelken JD, et al. MR evaluation of
CNS tumors: dose comparison study with gadopentetate
dimeglumine and gadoteridol. Radiology 1991; 180:485-491
10.Healy ME, Hasselink JR, Press GA, Middleton MS. Increased
detection of intracranial metastases with intravenous GdDTPA. Radiology 1987; 165:619-624
11.Akeson P, Vikhoff B, Stahlberg F, Holtas S. Brain lesion
contrast in MR imaging: dependence of field strength and
concentration of gadodiamide injection in patients and
phantoms. Acta Radiol 1977; 38:14-18
12.Runge VM. A review of contrast media research in 1999 –
- Invest Radiol 2001; 36:123-130
13.Yuh WT, Parker JR Carvlin MJ. Indication related dosing for
magnetic resonance contrast media. Eur Radiol 1977; 7(suppl
5):S269-S275
14.Rinck PA, Muller RN. Field strength and dose dependence of
contrast enhancement by Gadolinium-based MR contrast
agents. Eur Radiol 1999; 9:998-1004
15.Schorner W, Laniado M, Niendorf HP, Schubert C, Felix R.
Time-dependent changes in image contrast in brain tumors
after gadolinium-DTPA. AJNR Am J Neuroradiol 1986;
Evaluation between 3.0 T vs 1.5 T MRI in Detection of Brain Metastasis using Double Dose Gd-DTPA
- 107 –
7:1013-1020
16.Chang KH, Ra DG, Han MH, Cha SH, Kim HD, Han MC.
Contrast enhancement of brain tumors at different MR field
strengths: comparison of 0.5 T and 2.0 T. AJNR Am J
Neuroradiol 1994; 15:1413-1419
17.Nobauer-Huhmann IM, Ba-Ssalamah A, Mlynarik V, et al.
Magnetic resonance imaging contrast enhancement of brain
tumors at 3 tesla versus 1.5 tesla. Invest Radiol 2002; 37:114-
119
18.Galicich JH, Sundaresan N, Thaler HT. Surgical treatment of
single brain metastasis: evaluation of results by computerized
tomography scanning. J Neurosurg 1980; 53:63-67
19.Runge VM, Carollo BR, Wolf CR, Nelson KL, Gelblum DY.
Gd-DTPA: A review of clinical indications in central nervous
system magnetic resonance imaging. Radiographics 1989;
9:929-958
20.Earnest F 4th, Ryu JH, Miller GM, et al. Suspected non-small
cell lung cancer: Incidence of occult brain and skeletal
metastases and effectiveness of imaging for detection-Pilot
study. Radiology 1999; 211:137-145
21.Koutras AK, Marangos M, Kourelis T, et al. Surgical
management of cerebral metastases from non-small cell lung
cancer. Tumori 2003; 89:292-297
22.Soffietti R, Ruda R, Mutani R. Management of brain
metastases. J Neurol 2002; 249:1357-1369. Review
Neuro Second Opinion Catches Missed Brain Lesions Suggestive of MS
4/16/20
Malcolm’s brain MRI was initially read as completely normal. Upon getting a second opinion, it was discovered that 22 lesions suggestive of multiple sclerosis had been missed. Here is his story.
[Malcolm]
After 18 months of deteriorating health, with a multitude of neurological symptoms, I went to see a neurologist. I was ordered a standard brain MRI to help figure out what was going on. By this time, I had been researching my symptoms for a few months, and, in doing so, had developed a basic understanding of what I was looking for: T2 lesions.
When I received the results from my MRI, the report said everything was normal. I met with my neurologist, and he too said there was nothing abnormal about my scan. When I viewed the MRI myself, however, I found 4 spots that looked to me to be brain lesions. I brought them up to my doctor at my next appointment but was essentially laughed at.
It was quite unsettling to have something so concerning be shrugged off. After that appointment, I decided I wanted to switch to a new neurologist and get a second opinion on my MRI scan. I made an appointment with the new doctor, but it was going to be another 6 weeks before I could be seen. Fortunately, I found DocPanel. I submitted my MRI for review and, within 48 hours, I got my results back.
[Dr. Michael Rozenfeld]
Malcolm reached out to DocPanel due to concern for a missed diagnosis. He was reportedly having symptoms suspicious for multiple sclerosis but his MRI was initially read as completely normal. Based on my review of his MRI, the exam was not normal. Malcolm had significant findings that were certainly suggestive of, but not entirely specific for, multiple sclerosis.
[Malcolm]
My second opinion results were quite alarming – there were not 4 lesions, but 22. Twenty-two! They were small, but they were most definitely there. I took the second opinion report to my new neurologist, and now I am being considered for possible Primary Progressive Multiple Sclerosis. I am scheduled to get new MRIs in November to confirm or rule out MS and you can be sure I will be sending them to DocPanel for a second look!
I have found that the most important thing people in my shoes can do is be your own advocate. MS is such an odd and individual disease. – Malcolm
[Dr. Michael Rozenfeld]
Radiology exams can be misinterpreted for a number of reasons – this is unfortunately not uncommon. While MRI is not the only piece in the puzzle for MS diagnosis, it plays a significant role. A false negative diagnosis made off an MRI scan could lead the neurologist and patient down an incorrect path and delay an accurate diagnosis, or potentially miss it entirely.
While MRI is not the only piece in the puzzle for MS diagnosis, it plays a significant role. A false negative diagnosis made off an MRI scan could lead the neurologist and patient down an incorrect path and delay an accurate diagnosis, or potentially miss it entirely.
The positive findings on Malcolm’s exam and his new report provide additional essential information for his neurologist, allowing him (the neurologist) to combine these imaging findings with other test results and physical exam findings to come to an accurate diagnosis.
I would always suggest patients seek a second opinion for complex medical diagnoses, especially if the patient feels that something is being missed or that their concerns aren’t being addressed. This could mean setting up an appointment with a second neurologist or submitting your radiology exams for a second opinion to a company such as DocPanel, where they will be interpreted by subspecialty trained and certified neuroradiologists. I personally like to explain the imaging findings in layman’s terms, to ensure that the patient understands the disease process. I believe this empowers patients so that they can take an active role in ensuring the best decisions regarding their care are made.
[Malcolm]
After a few months of working with my current neurologist and a support group of people diagnosed with MS, I have found that the most important thing people in my shoes can do is be your own advocate. MS is such an odd, and individual disease. No two patients are exactly alike in their presentation or their symptoms. This can make it very difficult to diagnose. It is always helpful to get several opinions; your health is just to darn important.
DocPanel is committed to making sure every patient receives excellent care. If you would like an expert second opinion on your medical imaging scan from Dr.Rozenfeld or one of our other neuroradiology subspecialists, you can learn more here.
Evaluation between 3.0 T vs 1.5 T MRI in Detection of Brain Metastasis using Double Dose Gd-DTPA Woo-Suk Chung, M.D.1 , Tae-Sub Chung, M.D.1 , Hyung Jung Kim, M.D.2 , Chul Min Ahn, M.D.2 , Jae Hoon Lee, M.D.1 , Jin Hur, M.D.1 , Arthur Eung-Hyuck Cho, M.D.1 Purpose : Early detection of small brain metastases is important.
The purpose of this study was to compare the detectability of brain metastases according to the size between 1.5 T and 3.0 T MRI. Materials and Methods :
We reviewed 162 patients with primary lung cancer who were examined for TNM staging. After administration of double dose of Gd-DTPA, MR imaging was performed with SPGR by 3.0 T MRI and then with T1 SE sequence by 1.5 T MRI. In each patient, three readers performed qualitative assessment. Sensitivity, positive predictive value, and diagnostic accuracy were calculated in 3.0 T and 1.5 T MRI according to size. Using the signal intensity (SI) measurements between the metastatic nodules and adjacent tissue, nodule-to-adjacent tissue SI ratio was calculated. Results : Thirty-one of 162 patients had apparent metastatic nodules in the brain at either 1.5 T or 3.0 T MR imaging. 143 nodules were detected in 3.0 T MRI, whereas 137 nodules were detected at 1.5 T MRI. Six nodules, only detected in 3.0 T MRI, were smaller than 3.0 mm in dimension. Sensitivity, positive predictive value, and diagnostic accuracy in 3.0 T MRI were 100 %, 100 %, and 100 % respectively, and in 1.5 T MRI were 95.8 %, 88.3 %, and 85.1 % respectively. SI ratio was significantly higher in the 3.0 T MRI than 1.5 T MRI (p=0.025). Conclusion : True positive rate of 3.0 T MRI with Gd-DTPA was superior to 1.5 T MRI with Gd-DTPA in detection of metastatic nodules smaller than 3.0 mm. Index words :Magnetic resonance (MR), high-field-strength imaging Brain neoplasm, metastases Contrast medium Introduction Brain metastases present a poor prognosis suggesting a shortened survival time. Early diagnosis of brain involvement and determination of the number of metastases are important not only for quality of life but also for cost effectiveness (1-3).
The decision regarding a conservative versus a surgical approach depends on the number of brain metastases detected by radiologic means (1-3). A contrast-enhanced MRI has become the method of choice for visualization of brain metastases (1). High[1]dose gadolinium-enhanced MR examinations may have advantages over 0.1 mmol/kg examinations in detecting early and/or small metastases (3-7).
A delayed study can also increase the contrast (2). Comparing to the 1.5 T MRI with an axial T1-weighted spin echo sequence (SE), the higher field strength MR systems combined with a sequence of SPGR (spoiled gradient recalled acquisition in the steady state) and administration of a high dose of Gd-DTPA (gadopentetate dimeglumine) may also have advantages in detecting small metastases, although this has not yet been verified by clinical data. The purpose of this study is to compare the detectability of brain metastases classified according to the size of nodules, between thin slice SPGR of 3.0 T and conventional thick slice SE of 1.5 T MRI with the administration of a double dose of Gd[1]DTPA.
Materials and Methods Patients From December 2002 to February 2004, a total of 162 consecutive patients with primary lung cancer participated in our study.
The institutional review board approved our study, and informed consent was obtained from all patients regarding the potential risks of both the double dose of contrast medium and two assessments by MRI scanning on a 3.0 T and 1.5 T machine. After the study, 31 patients of this population were diagnosed as having brain metastases. Mean patient age was 61.2 (range, 43 to 80 years). Protocol On the 3.0 T MR scanner (GE Signa VH/i; GE medical system, Milwaukee, USA), the images were acquired using a standard head coil and an actively shielded gradient system with a maximum gradient strength of 43 mT/m. On the 1.5 T MR scanner (Magnetom Vision; Siemens Medical Systems, Erlangen, Germany), a standard head coil and a maximum gradient strength of 25 mT/m were used. All patients were examined after administrating a contrast agent with a double dose of Gd-DTPA (0.2 mmol/kg). First, examinations were performed on a 3.0 T MR scanner and then were subsequently performed on a 1.5 T MR scanner without additional contrast injection.
The scan interval between the 3.0 T and 1.5 T MR examination was less than 20 minutes. The contrast agent used was Gd-DTPA (Magnevist; Schering AG, Berlin, Germany). In all patients, the double dose of Gd-DTPA at 0.2 mmol/kg was administered intravenously as a bolus and scanning commenced immediately. MR imaging included the following sequences on both scanners:
At 3.0 T MRI, an axial 3D SPGR, which is usually used at present, was used (TR/TE/TI = 5.7/1.44/400 milliseconds; flip angle 20°; 2 mm slice thickness; FOV of 220 mm; matrix size of 512×512 ZIP; spatial resolution of 0.43×0.43×2 mm; two acquisition) with a scan time of 3 minutes 30 seconds. At 1.5 T MRI, an axial T1-weighted spin echo sequence was used (TR/RE = 600/14 milliseconds; flip angle 90°; 5 mm slice thickness; FOV of 210 mm; matrix size of 174×256; spatial resolution of 1.2×0.82×5 mm; two acquisition) with a scan time of 5 minutes 30 seconds.
Three radiologists performed randomized, independent blinded review. The three readers were merely informed that all of the patients had lung malignancies. The postcontrast MR examinations of the brain on all of the patients were evaluated. The readers did not have access to other image sets within each study. The presence, size, and number of metastatic nodules were assessed. The postcontrast images were divided into the following two groups: 3.0 T MRI with a double dose of Gd-DTPA and 1.5 T MRI with a double dose of Gd-DTPA. The readers were asked to document the number of nodules. Each nodule in each study was numbered and classified according to its largest diameter measurement: ≤3 mm, 3 mm to 5 mm, or >5 mm. Woo-Suk Chung et al – 102 – Subsequently, if there was any debate about nodule classification, a final interpretation using imaging studies was done until consensus among the readers was accomplished. If disagreements persisted, 3-6 months of follow-up MR scans were obtained for further validation.
For the patients who had follow-up MR scans, these scans were considered positive for metastases if there was a response to the treatment or if there was a growth of nodules identified during the follow-up period. In addition, the sensitivity, specificity, positive predictive value, negative predictive value, and diagnostic accuracy of the 3.0 T and 1.5 T MRI were calculated using the results from the final interpretation according to size. A quantitative image assessment was performed next. The same lesions on scans of the two different MRI systems were determined by comparing peripheral structures. Signal intensities of adjacent tissue and nodule were assessed by region of interest (ROI) measurements placed identically on both series of images using the same sized circular ROI from the software available on both scanners. Nodule-to[1]adjacent tissue contrast (SI ratio) is defined by SI ratio = ×100 Stissue1 taken as the signal intensity of an ROI assessed over a nodule and Stissue2 taken as the signal intensity assessed over the contralateral white matter.
The signal intensity of a nodule was measured within an enhanced area. In inhomogeneously enhanced nodules, the area of maximum uptake was chosen for measurement. Statistical Analysis The paired Wilcoxon’s signed ranks test was used to compare qualitative scores, and the matched-pair-t test was used to compare nodule SI ratios between the 3.0 T and 1.5 T MRI with a double dose of Gd-DTPA. For all tests, significance was set at p < 0.05, and SPSS software (SPSS Inc. Chicago, US) was used for statistical analyses. Results In the final review made by consensus of three radiologists (Table 1), a total of 143 metastatic nodules were detected by the 3.0 T MRI with a double dose of Gd-DTPA. Of these nodules, 49 nodules were ≤ 3 mm in diameter, 37 nodules were 3 mm to 5 mm, and 57 nodules were >5 mm. A total of 137 metastatic nodules were detected by the 1.5 T MRI with a double dose of Gd-DTPA in the final interpretation. Of these nodules, 43 nodules were ≤ 3 mm in diameter. The 3.0 T MRI with a double dose of Gd-DTPA was significantly more effective at detecting nodules smaller than 3 mm (p = 0.014) than the 1.5 T MRI. However, we found no significant difference in the detection of nodules larger than 3 mm (p > 0.05) (Table 1). In 6 of the 31 cases, six nodules, which were only detected by the 3.0 T MRI with a double dose of Gd[1]DTPA, and missed by the 1.5 T MRI with a double dose of Gd-DTPA, were unanimously agreed upon by all participants in the final interpretation. In three of the six nodules, a growing nodule was detected during a follow-up MR scan which confirmed the presence of metastases (Fig. 1). Three of the 6 nodules missed were unable to be detected using the 1.5 T MRI with a double dose of Gd-DTPA due to artifact (Fig. 2). Finally the 3.0 T MRI with a double dose of Gd-DTPA was Stissue1 – Stissue2 Stissue2 Evaluation between 3.0 T vs 1.5 T MRI in Detection of Brain Metastasis using Double Dose Gd-DTPA – 103 – Table 1. Total Number of Brain Metastases Classified by Size and Detected at the Final Interpretation Size 3.0 T MRI with 1.5 T MRI with p value Gd-DTPA Gd-DPTA ≤ 3 mm 49 43 0.014 3 mm<-≤5 mm 37 37 > 0.05 5 mm< 57 57 > 0.05 Total 143 137 0.014 Note : Data are the number of nodules Table 2. The Values of Currently Used Statistical Measures for the 3.0 T and 1.5 T MRIs with Gd-DTPA According to Nodule Size Metastatic nodules Total ≤ 3 mm Present Absent Present Absent 3.0 T MRI with Positive 143 00* 49 0* Gd-DTPA Negatiive 000 00* 00 0* 1.5 T MRI with Positive 137 18* 43 18* Gd-DTPA Negative 006 00* 06 0* * Pseudolesions that were detected as nodules by the 1.5 T MRI were detected as vascular structures by the 3.0 T MRI. useful for confirming the presence of these nodules. Nodules observed by the 3.0 T MRI with a double dose of Gd-DTPA were brighter or better delineation than by the 1.5 T MRI with a double dose of Gd-DTPA. Out of 137 nodules, 65 were brighter (Fig. 3) and out of 137 nodules, 63 were better delineated (Fig. 4). Forty[1]five of 137 nodules were satisfactory in both conditions. By consensus, the final review stated, nodules detected by the 3.0 T and 1.5 T MRI were considered positive for metastases (Table 2). Additionally, there were 18 pseudolesions which were detected as nodules by the 1.5 T MRI, but as vascular structures by the 3.0 T MRI (Fig. 5). The sensitivity, positive predictive values, and diagnostic accuracy of the 1.5 T MRI with a double dose of Gd-DTPA were 95.8%, 88.3%, and 85.1% respectively. The sensitivity, positive predictive value, and diagnostic accuracy of the 3.0 T MRI with a double dose of Gd-DTPA were 100%, 100%, and Woo-Suk Chung et al – 104 – a b Fig. 2.
A 73-year-old male with multiple brain metastases. Metastatic nodule (a) by a 3.0 T MR image with a double dose of Gd-DTPA show a metastatic nodule (arrow). But (b) the 1.5 T MR image with a double dose of Gd-DTPA cannot show the metastatic nodule by artifact. abc Fig. 1. A 65-year-old male with single brain metastasis. (a) The 3.0 T MR image with a double dose of Gd-DTPA shows a small metastatic nodule (arrow). (b) The 1.5 T MR image with a double dose of Gd-DTPA cannot show the nodule. In (c), a growing nodule was detected during the follow-up MRI with a double dose of Gd-DTPA which confirmed the presence of metastasis (arrow). 100% respectively. For metastatic nodules smaller than 3 mm, the sensitivity, positive predictive value, and diagnostic accuracy of the 1.5 T MRI with a double dose of Gd-DTPA were 87.8%, 70.5%, and 64.2% respectively. The sensitivity, positive predictive value, and diagnostic accuracy of 3.0 T MRI with a double dose of Gd-DTPA were 100%, 100%, and 100% respectively (Table 3). For quantitative image assessment, the SI ratio in the post contrast sequences (Table 4) was significantly higher in the 3.0 T MRI with a double dose of Gd[1]DTPA than in the 1.5 T MRI with a double dose of Gd[1]DTPA (p = 0.025). Discussion Detection of metastatic nodules is dependent on both their size and contrast ratio (2). As methods to increase the contrast, a higher dose of Gd-DTPA, a higher field strength, and delayed study can all be used to aid detection (2-5, 8-17). Nodules larger than 10 mm are easily detected because vasogenic edema is customarily associated with larger metastases (2). So, when a higher dose of Gd-DTPA is used, the detection rate of larger nodules is not influenced. However, a higher dose is helpful for detecting small nodules because it increases nodule enhancement, yet this method has the disadvantages of increasing the false positive rate and promoting side effects (2-5, 8-14).
A delayed study can also increase the contrast (2). It has been recommended that image acquisition be delayed from 5 to 35 minutes after the administration of contrast material at a dose of 0.1 mmol/kg to ensure optimal detection (2, 15). Three of the 6 nodules missed were unable to be detected using the 1.5 T MRI with a double dose of Gd[1]DTPA due to partial volume artifact. Eighteen lesions were detected in the 1.5 T MRI with a double dose of Gd-DTPA, which proved to be vascular structures in 3.0 T MRI with a double dose of Gd- DTPA. The true nature of these lesions was revealed because of the greater morphologic detail visualized by the high field Evaluation between 3.0 T vs 1.5 T MRI in Detection of Brain Metastasis using Double Dose Gd-DTPA – 105 – a b Fig. 3. A 65-year-old male with multiple brain metastases. (a) The 3.0 T MR image with a double dose of Gd-DTPA shows a metastatic nodule with spherical enhancement (arrow). (b) The 1.5 T MR image with a double dose of Gd-DTPA shows a metastatic nodule with spherical enhance[1]ment (arrow). Nodules observed by the 3.0 T MRI with a double dose of Gd-DTPA were brighter than when observed by the 1.5 T MRI with a double dose of Gd[1]DTPA. Table 3. Estimation of the Values of Currently Used Statistical Measures for the 3.0 T and 1.5 T MRI with Gd-DTPA According to Nodule Size Metastatic nodules Total ≤ 3 mm 3.0 T 1.5T 3.0 T 1.5T Sensitivity 100 95.8 100 87.8 Specificity – 0 – 0 Positive predictive value 100 88.3 100 70.5 Negative predictive value – 0 – 0 Diagnostic accuracy 100 85.1 100 64.2 Table 4. Results of a quantitative assessment of nodules that were detected by the 3.0 T and 1.5 T MRIs with Gd-DTPA (n=137) 3.0 T 1.5 T P value Nodules-to-adjacent tissue: SI ratio 94.59 63.86 0.025 strength MR image, thus allowing differentiation between true enhancing lesions and sulcal vessels. In our study, each nodules was classified according to its largest diameter as being ≤ 3 mm, 3 mm to 5 mm, and >5 mm because the slice thickness of the 3.0 T MRI was 2.0 mm and 5 mm for the 1.5 T MRI. Therefore this study classified small metastatic nodules as being smaller than 3 mm. Although our study supports the use of a higher field strength MRI with a double dose of Gd-DTPA for increased metastatic nodule detection and for improved nodule enhancement and delineation, the results should be interpretated with caution.
The reason for this is that for patients with two or more brain metastases, additional metastases found with the 3.0 T MRI seem to be of limited clinical importance. The presence of two or more small nodules generally will not change the way the patient is managed. Therefore, it is of utmost importance to identify the difference between none, one, and more than one metastatic nodule.
Patients with a single metastatic nodule located in a respectable region can be treated surgically, and the tumor staged as M1, not M0. However, patients with two or more metastatic nodules are usually Woo-Suk Chung et al – 106 – a b Fig. 5. A 67-year-old female with multiple brain metastases. (a) The 3.0 T MRI with a double dose of Gd-DTPA shows vascular structure (arrow). (b) The 1.5 T MRI with a double dose of Gd[1]DTPA shows a nodular lesion (arrow). A pseudolesion that was detected as a nodule by the 1.5 T MRI with a double dose of Gd[1]DTPA but as a vascular structure by the 3.0 T MRI with a double dose of Gd-DTPA is showed in this figure. a b Fig. 4. A 64-year-old male with multiple brain metastases. (a) The 3.0 T MRI with a double dose of Gd-DTPA shows a metastatic nodule with ring enhancement (arrow). (b) The 1.5 T MRI with Gd-DTPA show a metastatic nodule with ring enhancement (arrow). Nodules observed by the 3.0 T MRI with a double dose of Gd-DTPA were better delineated than when observed by the 1.5 T MRI with a double dose of Gd[1]DTPA. treated with radiation therapy and/or systemic chemotherapy (3, 18-22). On the other hand, the use of a higher strength field MRI was found to be helpful in confirming the appearance of an equivocal metastatic nodule. The 3.0 T MRI with a double dose of Gd-DTPA was also useful for the detection of additional metastases in patients with a known lesion detected by the 1.5 T MRI with a double dose of Gd[1]DTPA. Because this also has influence on the sensitivity, positive predictive value, and diagnostic accuracy the 3.0 T MRI with a double dose of Gd[1]DTPA was found to have better results than the 1.5 T MRI with a double dose of Gd-DTPA in our study. Therefore we recommend the use of the 3.0 T MRI with a double dose of Gd-DTPA in only three circumstances: when the findings by the 1.5 T MRI with Gd-DTPA are equivocal, when one potentially surgically respectable nodule is identified, or for detecting early and/or small metastases
There were two limitations in this study. The first limitation was a difference in protocol sequence. The T1 weighted spin-echo protocol was used for the 1.5 T MRI and SPGR technique protocol for the 3.0 T MRI. Detectability of metastatic nodules is more effective with 3.0 T MRI with T1 SE than with a 1.5 T MRI with SE (2). SPGR with thin slice thickness is superior to the T1 spin echo sequence with thick slice thickness due to a partial volume effect. Additionally, SE is not optimum for a 3.0 T due to longer T1- and shorter T2- relaxation times of water protons, which decrease the contrast ratio in the 3.0 T images.17 The purpose of this study was to detect early small brain metastases, so using the SPGR sequence with a 3.0 T MRI, which is usually used at present, has advantages for detecting early small brain metastases. The second limitation was that the scan interval between the 1.5 T and 3.0 T MRI was less than 20 minutes. However, a delayed study increases contrast as mentioned above. The current study found that the SI ratio was significantly higher in the 3.0T images than in the 1.5 T images. Considering the delayed study by the 1.5 T MRI, this limitation emphasizes the better detection rate of the 3.0 T MRI with a double dose of Gd-DTPA. And due to these confounding variables, blinded reviews were performed by three readers. The detectability of metastatic nodules smaller than 3mm was better using a 3.0 T MRI with SPGR than a 1.5 T MRI with T1 SE. Therefore we recommend a 3.0 T MRI with SPGR and a double dose of Gd-DTPA for detecting early and/or small metastatic nodules, furthermore influencing treatment. References 1.Lassman AB, DeAngelis LM. Brain metastases. Neurol Clin. 2003; 21:1-23, vii, Review 2.Ba-Ssalamah A, Nobauer-Huhmann IM, Pinker K, et al. Effect of contrast dose and field strength in the magnetic resonance detection of brain metastases. Invest Radiol 2003; 38: 415-422 3.Sze G, Johnson C, Kawamura Y, et al. Comparison of single[1]and triple-dose contrast material in the MR screening of brain metastases. AJNR Am J Neuroradiol 1998; 19:821-828 4.Runge VM, Kirsch JE, Burke VJ, et al. High-dose Gadoteridol in MR imaging of intracranial neoplasm. J Magn Reson Imaging 1992; 2:9-18 5.Yuh WT, Engelken JD, Muhonen MG, Mayr NA, Fisher DJ, Ehrhardt JC. Experience with high-dose gadolinium MR imaging in the evaluation of brain metastases. AJNR Am J Neuroradiol 1992; 13:335-345 6.Brekenfeld C, Foert E, Hundt W, Kenn W, Lodeann KP, Gehl HB. Enhancement of cerebral diseases: How much contrast agent is enough? Comparison of 0.1, 0.2, and 0.3 mmol/kg Gadoteridol at 0.2 T with 0.1 mmol/kg Gadoteridol at 1.5 T. Invest Radiol 2001; 36:266-275 7.Yuh WT, Tali ET, Nguyen HD, Simonson TM, Mayr NA, Fisher DJ. The effect of contrast dose, imaging time, and lesion size in the MR detection of intracerebral metastasis. AJNR Am J Neuroradiol 1998; 16:373-380 8.Van Dijk P, Sijens PE, Schmitz PI, Oudkerk M. Gd-enhanced MR imaging of brain metastases: contrast as a function of dose and lesion size. Magn Reson Imaging 1997; 15:535-541 9.Yuh WT, Fisher DJ, Engelken JD, et al. MR evaluation of CNS tumors: dose comparison study with gadopentetate dimeglumine and gadoteridol. Radiology 1991; 180:485-491 10.Healy ME, Hasselink JR, Press GA, Middleton MS. Increased detection of intracranial metastases with intravenous Gd[1]DTPA. Radiology 1987; 165:619-624 11.Akeson P, Vikhoff B, Stahlberg F, Holtas S. Brain lesion contrast in MR imaging: dependence of field strength and concentration of gadodiamide injection in patients and phantoms. Acta Radiol 1977; 38:14-18 12.Runge VM. A review of contrast media research in 1999 – 2000. Invest Radiol 2001; 36:123-130 13.Yuh WT, Parker JR Carvlin MJ. Indication related dosing for magnetic resonance contrast media. Eur Radiol 1977; 7(suppl 5):S269-S275 14.Rinck PA, Muller RN. Field strength and dose dependence of contrast enhancement by Gadolinium-based MR contrast agents. Eur Radiol 1999; 9:998-1004 15.Schorner W, Laniado M, Niendorf HP, Schubert C, Felix R. Time-dependent changes in image contrast in brain tumors after gadolinium-DTPA. AJNR Am J Neuroradiol 1986; Evaluation between 3.0 T vs 1.5 T MRI in Detection of Brain Metastasis using Double Dose Gd-DTPA – 107 – 7:1013-1020 16.Chang KH, Ra DG, Han MH, Cha SH, Kim HD, Han MC. Contrast enhancement of brain tumors at different MR field strengths: comparison of 0.5 T and 2.0 T. AJNR Am J Neuroradiol 1994; 15:1413-1419 17.Nobauer-Huhmann IM, Ba-Ssalamah A, Mlynarik V, et al. Magnetic resonance imaging contrast enhancement of brain tumors at 3 tesla versus 1.5 tesla. Invest Radiol 2002; 37:114- 119 18.Galicich JH, Sundaresan N, Thaler HT. Surgical treatment of single brain metastasis: evaluation of results by computerized tomography scanning. J Neurosurg 1980; 53:63-67 19.Runge VM, Carollo BR, Wolf CR, Nelson KL, Gelblum DY. Gd-DTPA: A review of clinical indications in central nervous system magnetic resonance imaging. Radiographics 1989; 9:929-958 20.Earnest F 4th, Ryu JH, Miller GM, et al. Suspected non-small cell lung cancer: Incidence of occult brain and skeletal metastases and effectiveness of imaging for detection-Pilot study. Radiology 1999; 211:137-145 21.Koutras AK, Marangos M, Kourelis T, et al. Surgical management of cerebral metastases from non-small cell lung cancer. Tumori 2003; 89:292-297 22.Soffietti R, Ruda R, Mutani R. Management of brain metastases. J Neurol 2002; 249:1357-1369. Review Woo-Suk Chung et al – 108 – 통신저자 : 정태섭, 서울특별시 강남구 도곡동 146-92 연세대학교 영동세브란스병원 영상의학과 Tel. 82-2019-3514 Fax. 82-3462-5472 E-mail: tschung@yumc.yonsei.ac.kr 뇌전이종양의발견에있어서Doble dose Gd-DTPA를이용한 3 T MRI와1.5 T MRI간의비교연구 1 연세대학교 영동세브란스병원 영상의학과 2 연세대학교 영동세브란스병원 호흡기내과 정우석1 ·정태섭1 ·김형중2 ·안철민2 ·이재훈1 ·허 진1 ·조응혁1 목적: 작은 뇌전이 종양의 조기 발견은 중요하다. 이 연구의 목적은 1.5 T MRI와 3.0 T MRI 간의 크기에 따른 뇌 전이 종양의 발견율을 비교하는 것이다. 대상 및 방법: 폐암으로 진단 받은 162명의 환자를 대상으로 TNM 병기를 위해 뇌 MRI를 시행하였다. Gd-DTPA 를 2배 용량으로 투여 후, 3.0 T MRI에서 훼손경사회복획득으로 촬영하였으며 그 후 1.5 T MRI에서 T1 스핀 에 코로 촬영하였다. 3명의 방사선과 전문의가 합의하여 MRI를 판독하였으며 정성 평가를 시행하였다. 3.0 T와 1.5 T MRI에서 크기에 따라 민감도, 양성 예측률, 정확도를 평가하였다. 신호 강도를 사용하여 전이 종양과 인접 조직간 의 신호강도 비를 계산하였다. 결과: 162명의 환자 중 31명에서 1.5 T 또는 3.0 T MR에 뇌전이 종양이 발견되었다. 3.0 T MRI에서 143개의 종 양이 발견되었으나 1.5 T MRI에서 137개의 종양이 발견되었다. 6개의 종양이 3.0 T MRI에서만 발견되었으며 크 기는 모두 3 mm 미만이었다. 3.0 T MRI의 민감도, 양성 예측률, 정확도는 각각 100%, 100%, 100%이며, 1.5 T MRI에서는 각각 95.8%, 88.3%, 85.1% 이다. SI ratio는 1.5 T MRI보다 3.0 T MRI에서 유의하게 높았다 (p=0.025). 결론: Double dose Gd-DTPA를 이용한 3.0 T MRI는 3 mm미만의 뇌전이 종양을 발견하는데 있어서 1.5 T
